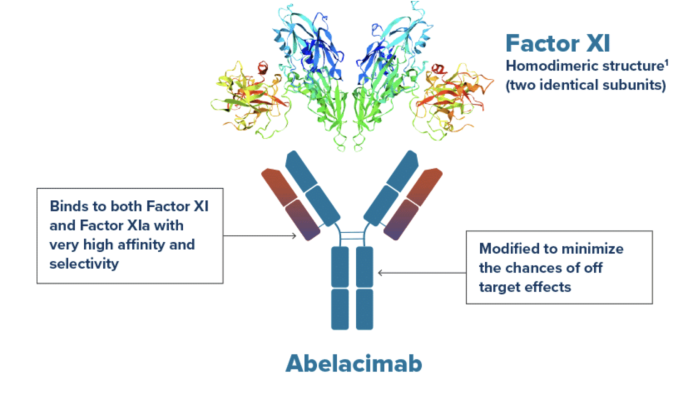
September 18, 2023 — Anthos Therapeutics, Inc., a clinical stage company developing innovative therapies for cardiovascular diseases, founded by Blackstone Life Sciences, announced today that the AZALEA-TIMI 71Phase 2 study in 1,287 patients with atrial fibrillation at moderate-to-high risk of stroke, met its primary endpoint. The study has been stopped early by the Data Monitoring Committee due to an overwhelming reduction in the composite of major and clinically relevant non-major bleeding in patients taking abelacimab compared with patients taking rivaroxaban, a leading standard-of-care DOAC. In addition, abelacimab is the first and only Factor XI inhibitor to demonstrate an unprecedented reduction in major bleeding compared to a DOAC, which is the most serious type of bleeding. Full results of AZALEA-TIMI 71 will be presented at an upcoming scientific congress. “The AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to the standard-of-care anticoagulant. With a median of 21 months of follow-up, spanning more than 2,000 patientyears, AZALEA-TIMI 71 represents a landmark study confirming the promise of Factor XI inhibition as causing substantially less bleeding than a current standard-of-care,” said Marc S. Sabatine, MD, MPH, the Lewis Dexter, MD, Distinguished Chair in Cardiovascular Medicine, Brigham and Women’s Hospital; Professor, Harvard Medical School; and Chairman of the Thrombolysis in Myocardial Infarction (TIMI) Study Group. Abelacimab is a novel, highly selective, fully human monoclonal antibody with dual inhibitory activity against Factor XI and its active form, Factor XIa. Abelacimab 150 mg maintains ~98% inhibition over the dosing interval, recapitulating the benign bleeding profile of patients with genetic Factor XI deficiency. Prior to AZALEA-TIMI 71, abelacimab achieved a ~80% reduction in venous thromboembolism (VTE) versus a standard-of-care comparator in a gold standard proof-of-concept efficacy study that was published in the New England Journal of Medicine. 1 “Given AZALEA-TIMI 71’s overwhelming reduction in bleeding, together with an 80% reduction in thrombosis demonstrated in our earlier VTE study, 1 abelacimab embodies its promise as a hemostasis-sparing anticoagulant and represents a paradigm shift in the prevention of stroke and other thrombotic conditions,” said Dan Bloomfield, MD, Chief Medical Officer of Anthos Therapeutics. “If approved, more patients with atrial fibrillation could be treated effectively and safely, with a much lower risk of bleeding with abelacimab as compared to a DOAC.” The Center for Disease Control and Prevention (CDC) estimates that 12.1 million people in the United States will have atrial fibrillation by 2030. 2 Unfortunately, 40% to 60% of patients with atrial fibrillation are not prescribed anticoagulants today. This underuse of anticoagulants for stroke prevention has been cited as one of the greatest public health issues facing cardiovascular patients. 3 In a physician survey, the foremost barrier to patients taking oral anticoagulants was bleed related. 4 “Abelacimab has the potential to provide a game-changing treatment option for all those patients who live with the daily fear of bleeding while taking current anticoagulants. We can now imagine a future where these patients are able to resume and enjoy activities that they are currently being forced to give up due to concerns associated with bleeding,” said Leslie Lake, President of the National Blood Clot Alliance. “We are thrilled that AZALEA-TIMI 71 has demonstrated such a positive outcome and excited about the promise that it offers to patients.”
For more information: http://anthostherapeutics.com/
References:
- Verhamme P et al. New Engl J Med July 2021 (https://www.nejm.org/doi/full/10.1056/NEJMoa2105872)
- 2 Center for Disease Control and Prevention website; Atrial Fibrillation page (https://www.cdc.gov/heartdisease/atrial_fibrillation.htm)
- Pokorney et al, American Heart Journal, April 2019 (https://www.sciencedirect.com/science/article/abs/pii/S0002870318302989?via%3Dihub)
- 4 Yao C et al PEC Innov 2022;1:100062. (https://www.sciencedirect.com/science/article/pii/S2772628222000474)
- 5 Yi BA et al. J Thromb Haemost. Oct. 2021 (https://pubmed.ncbi.nlm.nih.gov/34714969/)
- 6 Hsu et al. J Am Coll Cardiol. Aug. 2021 (https://www.sciencedirect.com/science/article/abs/pii/S0735109721053213?via%3Dihub)
Related Content:
Gender Differences and AFib: New Study Flips Conventional Wisdom
Global AFib Study Finds Simple Approach is Best for Ablation Procedures


 December 19, 2025
December 19, 2025 









