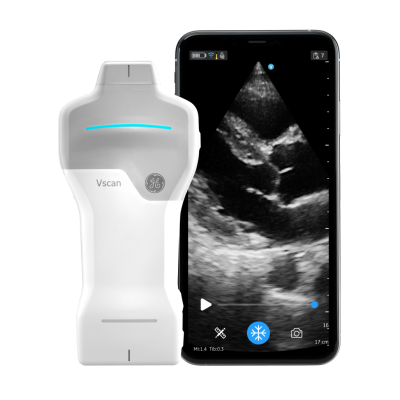
GE HealthCare announced the launch of a handheld, wireless ultrasound imaging system designed for rapid cardiac and vascular assessments at the point of care during the recent European Society of Cardiology (ESC) 2023 meeting held Augsut 25-28 in Amsterdan, The Netherlands. Image courtesy: GE HealthCare
September 5, 2023 — GE HealthCare announced the launch of a handheld, wireless ultrasound imaging system designed for rapid cardiac and vascular assessments at the point of care during the recent European Society of Cardiology (ESC) 2023 meeting held Augsut 25-28 in Amsterdan, The Netherlands. The latest addition to the Vscan product suite, Vscan Air SL was designed to help clinicians accelerate diagnoses and treatment decisions, according to a company statement.
The device features GE HealthCare’s proprietary SignalMax and XDclear technology that provide high levels of penetration, resolution, and sensitivity in imaging performance with an industry leading single crystal transducer technology. The portable, wireless Vscan Air SL is designed to enable clinicians to efficiently collect and view crystal clear cardiac and vascular images at the point of care. By streamlining these workflows and avoiding overloading traditional radiology resources, clinicians can expedite care decisions to help patients receive treatment plans right away when time is of the essence, noted the company.
“Having ever more powerful handheld ultrasound is a game changer for patient care,” said Guy Lloyd, M.D., FRCP, consultant cardiologist at Barts Heart Center, University College London Hospitals and clinical director for Cardiovascular Diagnostics and Investigations. He added, “Whether on the ward or in general practice, being able to provide high-quality imaging at the point of care means rapid diagnosis and rapid treatment.”
Vscan Air SL provides clinicians with a pocket-sized, portable tool that allows for clear, whole-body scanning and secure viewing of images. Through Vscan Air + Digital Tools, clinicians have access to subscriptions that can connect them to a suite of easy-to-use solutions designed to improve workflow with secure collaboration, image, and device management features, noted the company. Featuring a dual-headed probe, the Vscan Air SL offers a sector array and a linear array on a single device which is ideal for switching between focused cardiac assessments and vascular assessments right at the point of care – both inside and outside of the hospital.
Vscan Air SL is currently commercially available in key countries throughout Europe and Asia as well as Australia and New Zealand. In the United States, Vscan Air SL is 510(k) cleared by the U.S. Food and Drug Administration and will become commercially available this quarter, according to the August 25 statement issued by the company.
For more information: gehealthcare.com


 June 08, 2023
June 08, 2023 

