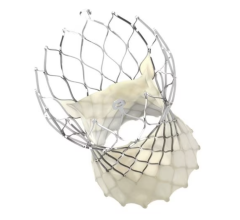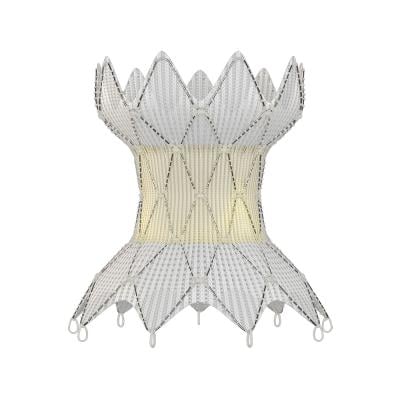
May 24, 2023 — Medtronic announced two-year results of their Harmony Transcatheter Pulmonary Valve (TPV) System, which treats severe pulmonary regurgitation (PR) in the native or surgically repaired right ventricular outflow tract (RVOT). The analysis demonstrates strong clinical and hemodynamic outcomes for patients with a congenital heart defect of the RVOT and was presented at the Society for Cardiovascular Angiography & Interventions (SCAI) 2023 Scientific Sessions.
“Sharing these longer-term outcomes for the Harmony Transcatheter Valve is an important milestone to help offer more streamlined treatment options for patients living with congenital heart disease,” Mary Hunt Martin, MD, FSCAI, director of Adult Congenital Intervention at University of Utah and Primary Children’s Hospital in, Salt Lake, Utah. “The strong safety profile reflected in these findings is especially encouraging since prior to Harmony, many of these patients would have to undergo multiple surgeries early on in their life.”
Congenital heart disease (CHD) is the most common type of birth defect in the United States, affecting an estimated 40,000 infants each year[1] and 1.6 million who live with the disease[2]. Approximately one in five patients born with CHD have structural malformations that disrupt the connection between the heart and the lungs[3], or the RVOT. The current standard of care is open-heart surgery or other interventions early in life to address these malformations. For the 80% of CHD patients who require a native or surgically repaired RVOT at birth, many will need a pulmonary valve replacement later in life, which often requires another open-heart surgery.
Harmony TPV was designed to treat patients with RVOT anomalies with severe pulmonary valve regurgitation (PR), a condition where blood leaks back into the right lower chamber of the heart after being pumped into the lungs. The Harmony TPV provides these patients with a minimally invasive treatment alternative.
Patients received a commercially available Harmony valve – a 22 mm valve (TPV22 device) or a 25 mm valve (TPV25 device)– as part of the Harmony Native Outflow Tract Early Feasibility Study (EFS), Harmony TPV Pivotal Trial, and Continued Access Study (CAS). Eligible patients had severe PR by echocardiography or PR fraction ≥30% by cardiac magnetic resonance imaging and a clinical indication for pulmonary valve replacement. In the study, 86 patients were implanted with a TPV22 (n=42) or TPV25 (n=44) device, all of whom remained implanted for >24 hours.
Key findings include 0% vascular injury requiring intervention, 99% freedom from major stent fracture, and 99% with no/trace PR at 2- years.
“These findings help deepen our long-term evidence for Harmony TPV and underscore our commitment to providing solutions for congenital patients with complex anatomies,” said Nina Goodheart, SVP and President of the Structural Heart & Aortic business at Medtronic. “Providing a system designed to reliably treat pulmonary regurgitation is an important way Medtronic innovations positively impacts this vulnerable patient population.”
The Harmony TPV System received U.S. FDA approval in 2021 based on the Harmony TPV clinical study that demonstrated safety and effectiveness. The Harmony TPV device is commercially available for use in the United States and Japan, and has received regulatory approval in Canada and Saudi Arabia. Earlier this year, Medtronic relaunched the Harmony TPV system following a voluntary recall of the Harmony Delivery Catheter System (DCS) in March 2022. Medtronic worked collaboratively with the FDA to remediate the issue and earn FDA approval to return the device to market.
For more information: www.medtronic.com
Find related SCAI23 conference coverage here
Recent Technology Advances in Congenital Heart:
FDA Clears First Device to Treat Right Ventricular Outflow Tract Congenital Heart Disease
VIDEO: Harmony Transcatheter Pulmonary Valve Has Good Outcomes at 1 Year — Interview with Tom Jones, M.D.
FDA Expands Indication for Melody Transcatheter Valve for Failed Surgical Valves
VIDEO: Use of Virtual Reality to Aid Congenital Heart Disease — Interview with David M. Axelrod, M.D.
Bioresorbable Pulmonary Valve Replacement May Enable Cardiovascular Regeneration
VIDEO: Transcatheter Closure of Holes in the Heart — Interview with Ziyad Hijazi, M.D.
Nemours Children's Health System Uses 3-D Printing to Deliver Personalized Care
Bioresorbable ASD Occluder Prepares to Enter U.S. Clinical Trial
FDA Approves Abbott's Amplatzer Piccolo Occluder
Critical Need for Pediatric Electrophysiology Devices is Focus of Medical Device Competition
Lab-created Heart Valves Can Grow With the Patient
Abbott Receives European CE Mark for Two Pediatric Heart Devices
ASE Releases Guidelines for Transesophageal Echo in Congenital Heart Disease


 May 05, 2025
May 05, 2025