February 24, 2023 — Biotronik announced that it has received CE approval for the Selectra 3D implant tools to include left bundle branch area pacing (LBBAP) in addition to His-bundle pacing (HBP). Commonly referred to as conduction system pacing (CSP) these two approaches have emerged as a physiologic pacing alternative to avoid dyssynchronous contraction of the heart which can induce various negative long-term effects for patients.1-3
Approved in 2021 for HBP, Selectra 3D’s safety and effectiveness for CSP has been shown in over 10 studies with more than 1,000 patients. In the multi-center Belgium registry that enrolled 353 patients in eight centers, the use of Selectra 3D for LBBAP showed 95% implant success, low pacing thresholds (0.6 +/- 0.4V at 0.4 ms) that remained stable at 12 months, and a low lead revision rate of 1.4%.4 "With this study we were able to show that left bundle branch area pacing using BIOTRONIK tools is a safe and reliable treatment option. It is associated with high implant success, low pacing thresholds and high safety profile," commented the leading study author Prof. Dr. Jan De Pooter, Cardiology and Electrophysiology at the University Hospital Ghent, Belgium.
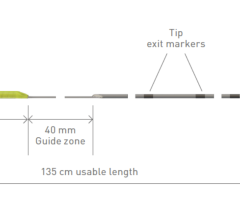
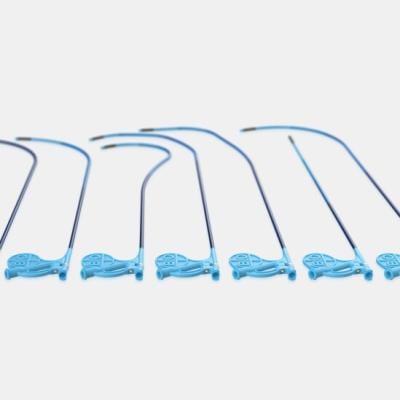
BIiotronik CSP tools include an extensive portfolio of catheters with nine distinct length and curve options that adapt to different patient anatomy. The effectiveness of these different variants was confirmed in a previous study5 showing that the 55mm curve fits for most anatomies, the 40mm curve allows to reach more proximal septal targets, and the 65mm curve more distal septal targets in dilated hearts. Enabling more choices for different patient anatomies, Selectra 3D supported over 90% of CSP procedures successfully. The electrical parameters were optimal at implant and remained stable over a median follow-up of 178 (34-402) days.
"Both clinical practice and intensive scientific research show the growing role of conduction system pacing. With our extensive CSP product line, we provide the tools that can benefit millions of patients in need of cardiac pacing," said Dr. Andreas Hecker, President CRM/EP at Biotronik.
For more information: www.biotronik.com


 January 29, 2026
January 29, 2026