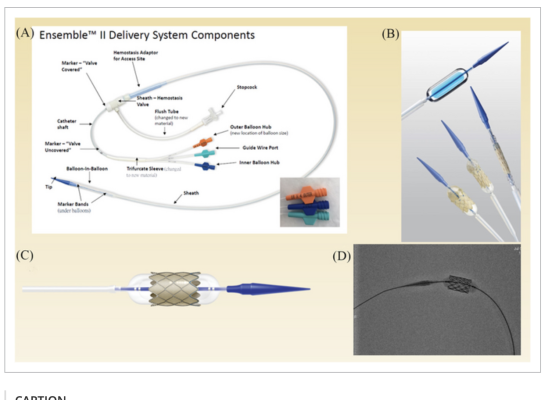
The ensemble delivery system of the Melody valve.
January 6, 2023 — Patients with congenital heart diseases often suffer from obstructions in the right ventricular outflow tract (RVOT), which carries blood from the heart to the lungs. These obstructions can be repaired with surgical procedures. However, these are temporary fixes that can still result in pulmonary stenosis and/or regurgitation, eventually leading to RVOT dysfunction and even fatal arrhythmias. Often, heart patients require pulmonary valve replacement (PVR) surgery; using bioprosthetic valves made from materials derived from cows or pigs, which deteriorate over time. This results in patients needing valve replacement surgeries throughout their lives, which increases the risk of surgical complications. To minimize the need for multiple surgeries and their resultant risks, medical researchers have been trying to find robust alternatives to surgical PVR. To this end, transcatheter PVR (TPVR) has emerged as a potential non-surgical option to restore RVOT and pulmonary valve functions without the concomitant surgical risks.
In this review article, Professor Adolphus Kai-Tung Chau from the Chinese University of Hong Kong Medical Centre discusses the benefits, challenges, clinical considerations, outcomes, and developments in the application of TPVR. The article was made available online on December 5, 2022, in an Early View version of Pediatric Investigation. “Different cardiology societies may adopt slightly different criteria for TPVR for both symptomatic and asymptomatic patients. But given that this procedure is minimally invasive compared to surgical PVR, it is likely that more asymptomatic patients may benefit from it,” says Prof. Chau.
The TPVR procedure requires a thorough pre-procedural evaluation, including patient history, physical examination, chest radiographs, electro- and echo-cardiograms, and imaging tests to ascertain the morphology of the RVOT and the presence of existing pulmonary complications. The surgical plan for individual patients is ideally determined by a multidisciplinary team of specialists.
Before the procedure, anatomical and hemodynamic data are assessed along with the assessment of the coronary artery and aorta. Next, the RVOT conduit is prepared for the implantation with pre-stenting and serial balloon dilation to ensure adequate pressure in the right ventricle and avoid valve failure.
What makes the TPVR procedure minimally invasive? Unlike conventional open-heart surgery, TPVR is conducted by guiding a hollow transcatheter tube through the femoral or jugular vein into the heart. The valve is implanted and set in position via balloon dilation. Following the implant, post-procedure hemodynamic data and angiograms are obtained to assess valve competence and check for perivalvular leak.
An advantage of the TPVR is that it can also be safely used for valve-in-valve conditions, where a new transcatheter valve is placed inside the hole of the failed valve after minor alterations.
Lately, trials are being conducted to adapt the application of TPVR for valve implantation in native, large, or variably shaped RVOTs, through self-expanding, partially covered stents that can fit into a wide range of RVOT shapes and sizes.
As of now, the Harmony and Edward Sapien valves are the only TPVR-compatible valves approved by the United States Food and Drug Administration (FDA). However, newer valves are continuously being developed and tested with promising results.
Long-term results indicate that TPVR can effectively restore RVOT function, while improving survival rates and reducing the need for reintervention across age groups. Nevertheless, vigilant testing is recommended to avoid endocarditis and coronary artery compression, which are rare but serious complications of TPVR. Although TPVR and surgical PVR have similar outcomes in terms of mortality rates and cardiovascular readmissions, TPVR leads to shorter hospital stays that benefit both patients and their caregivers.
In conclusion, this less invasive procedure is capable of providing safe and durable long-term outcomes, which makes it an important and preferred option for patients with congenital heart diseases. “TPVR is constantly evolving and undergoing improvement in efficacy and safety. With more long-term results from trials and better delivery systems, TPVR might also be useful as an initial repair option for younger and growing children,” envisions Prof. Chau.
For more information: www.pediatricinvestigation.org
Recent Technology Advances in Congenital Heart:
FDA Clears First Device to Treat Right Ventricular Outflow Tract Congenital Heart Disease
VIDEO: Harmony Transcatheter Pulmonary Valve Has Good Outcomes at 1 Year — Interview with Tom Jones, M.D.
FDA Expands Indication for Melody Transcatheter Valve for Failed Surgical Valves
VIDEO: Use of Virtual Reality to Aid Congenital Heart Disease — Interview with David M. Axelrod, M.D.
Bioresorbable Pulmonary Valve Replacement May Enable Cardiovascular Regeneration
VIDEO: Transcatheter Closure of Holes in the Heart — Interview with Ziyad Hijazi, M.D.
Nemours Children's Health System Uses 3-D Printing to Deliver Personalized Care
Bioresorbable ASD Occluder Prepares to Enter U.S. Clinical Trial
FDA Approves Abbott's Amplatzer Piccolo Occluder
Critical Need for Pediatric Electrophysiology Devices is Focus of Medical Device Competition
Lab-created Heart Valves Can Grow With the Patient
Abbott Receives European CE Mark for Two Pediatric Heart Devices
ASE Releases Guidelines for Transesophageal Echo in Congenital Heart Disease


 May 19, 2025
May 19, 2025