November 7, 2022 — HeartFlow, Inc., the leader in revolutionizing precision heartcare, today announced new trial results that show evaluating patients with suspected CAD by applying advanced artificial intelligence (AI) to CCTA allows for more accurate non-invasive diagnosis, reduces unnecessary testing, and offers higher confidence in identifying patients needing treatment.
The results from the Prospective Randomized Trial of the Optimal Evaluation of Cardiac Symptoms and Revascularization (PRECISE) trial were presented today as late-breaking clinical data at the American Heart Association’s (AHA) Scientific Sessions in Chicago, IL.
“This is the strongest level of scientific evidence yet to support the approach professional guidelines recommend that physicians use to diagnose stable chest pain patients,” said Pamela Douglas, M.D., MACC, FASE, FAHA, the Ursula Geller Professor for Research in Cardiovascular Disease, Duke University School of Medicine and study chair of the PRECISE trial. “Combining multiple steps into a Precision Pathway provides a clear path forward for clinicians.”
PRECISE is the first global randomized controlled trial of its kind, enrolling 2,103 participants at 65 sites. The trial confirms that the CCTA ± FFRCT-centered strategy, recognized by the AHA/ACC Guidelines, is the superior diagnostic pathway for patients with suspected coronary artery disease.
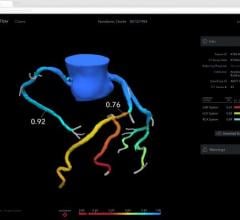
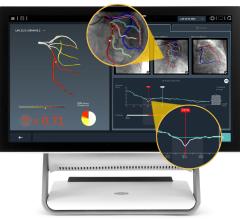
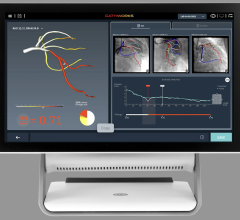
The trial compares a non-invasive Precision Pathway to Traditional Testing which includes stress testing or invasive cardiac catheterization. The Precision Pathway is consistent with the AHA/ACC guideline-recognized approach that defers testing for low-risk patients and tests all others with CCTA ± FFRCT. The FFRCT Analysis uses AI coupled with highly trained analysts to create an interactive 3D computer model of the heart that quantifies and displays blood flow and blockages.
Compared to Traditional Testing, the PRECISE trial showed that the Precision Pathway:
- allows for more accurate non-invasive diagnosis, significantly lowering the rates of false negatives and false positives compared to Traditional Testing in patients with coronary artery disease.
- reduces unnecessary tests, providing a better patient experience, with a 4x reduction in unnecessary invasive catheterization and necessitating fewer initial diagnostic tests overall.
- provides confidence in treating the right patients - 75% more likely to identify patients in need of intervention.
“We’re pleased to see these data support what the guidelines already recognize,” said Campbell Rogers, MD, FACC, Chief Medical Officer, HeartFlow. “We believe this level-one clinical science information will be used by more practitioners to guide decisions on how to optimize care for people with suspected coronary artery disease.”
A CCTA ± FFRCT pathway has been adopted by more than 725 hospitals worldwide, including 80% of the Top 50 Heart Hospitals in the US.
For more information: www.heartflow.com
Find more AHA conference coverage here
Related PRECISE Trial Content:
Processed Fat Stem Cells Show Potential for Refractory Ischemia Patients


 February 03, 2026
February 03, 2026