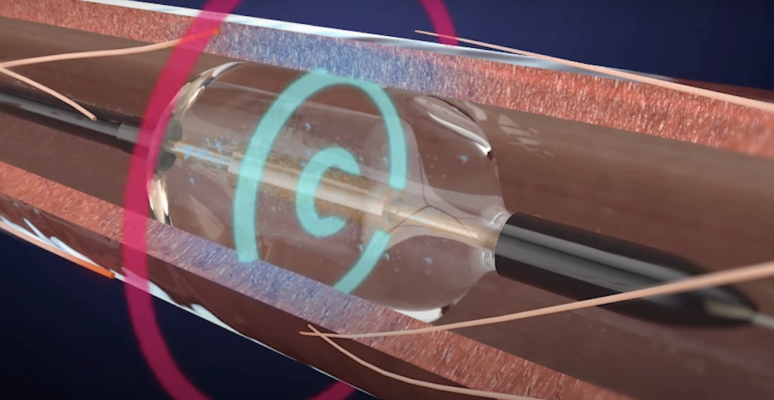
September 26, 2022 — ReCor Medical, Inc., a wholly owned subsidiary of Otsuka Medical Devices Co., Ltd., announced the detailed results from the RADIANCE II US FDA IDE pivotal trial evaluating the endovascular Paradise Ultrasound Renal Denervation (uRDN) System as a treatment for hypertension. Principal Investigator Ajay J. Kirtane, MD, Professor of Medicine at Columbia University, Vagelos College of Physicians and Surgeons / NewYork-Presbyterian Hospital, presented the study results in a Late Breaking Clinical Science session at TCT 2022, the annual scientific symposium of the Cardiovascular Research Foundation. The highly anticipated results follow ReCor’s announcement in July that the RADIANCE II study met its primary efficacy endpoint, demonstrating a statistically significant reduction in daytime ambulatory systolic blood pressure at two months between uRDN and a sham procedure.
Conducted as an international, multicenter study, RADIANCE II is a US FDA IDE, randomized, sham-controlled pivotal trial of the Paradise uRDN System in the treatment of patients with uncontrolled hypertension. Among 1038 patients screened for eligibility at more than 60 study centers in 8 countries, 224 patients with uncontrolled hypertension were randomized 2:1 to uRDN or a sham. Patients were to remain off antihypertensive medications throughout the 2 months of follow-up unless specified BP criteria were exceeded. At the 2-month primary efficacy endpoint, patients treated with the Paradise uRDN system had a mean reduction in daytime ambulatory systolic blood pressure of -7.9 mmHg, compared to a reduction of -1.8 mmHg in the sham arm, corresponding to a statistically significant between-group difference of -6.3 mmHg (p<0.0001). Similar reductions in blood pressure were observed in nighttime and 24-hour measures, as well as measurements taken at home and in the physician office. No major adverse events were seen at 30 days, the primary safety endpoint will be measured at 6 months, and patients will be followed for 60-months.
“These results are important to the field of hypertension treatment. RADIANCE II is the third and largest randomized, sham-controlled study to show that the Paradise uRDN System delivers meaningful reductions in blood pressure in patients with uncontrolled hypertension,” said Study Principal Investigator Ajay Kirtane. “On behalf of my co-principal investigator Professor Michel Azizi and the entire steering committee, I would like to thank the study patients, investigators, and coordinators who gave so much of themselves—including during the COVID pandemic—in order to complete this rigorously conducted trial.”
Echoing these thoughts, study principal investigator Michel Azizi, Professor of Medicine at Université Paris Cité, Hôpital Européen Georges Pompidou, Paris, France, said, “The results from RADIANCE II provide further evidence for uRDN as a potential therapy option for hypertension. The RADIANCE II results are strongly consistent across all measures of blood pressure within the study and are also consistent with the prior SOLO (off-medication) and TRIO (on triple antihypertensive combination treatment) trials—adding confidence in the treatment effect of the Paradise uRDN system across a broad spectrum of hypertension severity. These results align well with the recent consensus statement from the European Society of Cardiology, supporting the use of renal denervation for treatment of uncontrolled hypertension. Additionally, if these results are maintained over the long-term—as already shown by the 36-month results of the SOLO trial and 24-month results of the TRIO trial—the reductions in blood pressure seen in the RADIANCE trials are of a magnitude previously shown in hypertension drug trials to be associated with cardiovascular risk reduction.”
“We are thrilled with the results from RADIANCE II. This is further evidence that the Paradise uRDN System lowers blood pressure in a wide range of patients who are struggling to control their hypertension,” said ReCor president and CEO, Andrew M. Weiss. “ReCor looks forward to working with physicians and regulating bodies around the world to make the Paradise System treatment available to patients and their physicians who are seeking better control of their hypertension.”
For more information: http://www.recormedical.com/


 October 31, 2025
October 31, 2025 









