June 27, 2022 — Acutus Medical, Inc., an arrhythmia management company focused on improving the way cardiac arrhythmias are diagnosed and treated, today announced the commercial launch of an expanded suite of left-heart access products to now include the AcQCross Qx system for use with the TruSeal and FX delivery system for the Watchman LAAC Device.
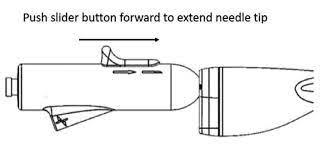
Gaining access to the left atrium requires physicians to cross the septum, a multi-step process that often involves the exchange of wires and needles while trying to achieve the proper angle and location on the septum. AcQCross is the first and only transseptal system engineered with an integrated needle and dilator to reduce these exchanges. US Left-atrial appendage closure procedures are expected to total over 50,000 in 2022, according to the Millennium Research Group. With this clearance, Acutus now offers sheath-compatible transseptal access devices that cover 409,000 electrophysiology and structural heart procedures in the US.
“Crossing the septum at the proper location is important when doing any left-sided heart procedure, but it can be especially critical to the success of delivering Watchman to the left atrial appendage,” said Dr. Tom Waggoner, DO, FACC, FSCAI, FSVM, RPVI, Director, Structural Heart Program and Cardiovascular Research, Tucson Medical Center, Tucson, Arizona. “With AcQCross, I can easily reposition without withdrawing or exchanging needles or wires, so its new compatibility with Watchman has made my procedures much safer for my patients and far more efficient for me and my team.”
AcQCross features an array of catheters that are length-, diameter- and tip-matched and designed to lock into the hub of market-leading sheaths used in the vast majority of left-heart procedures, including delivery of the Watchman. With the expanded product offering, physicians can utilize AcQCross with their preferred sheaths during virtually any left-heart access procedure.
“The AcQCross system provides interventional cardiologists and electrophysiologists with unique benefits of broad compatibility with market-leading access sheaths while also enhancing procedure versatility and workflow,” said David Roman, interim Chief Executive Officer and Chief Financial Officer, Acutus Medical. “The expanded AcQCross product line allows us to bring this innovative technology to a wider range of procedure categories that should drive sustained growth in this portfolio.”
AcQCross and its entire line of catheters are commercially available in the United States.
For more information: www.acutusmedical.com/us/.
Related content:
Late-Breaking Study Results Reinforce Positive Real-World Outcomes with the WATCHMAN FLX LAAC Device
PINNACLE FLX Study of New Watchman FLX LAA Occluder Meets Safety and Efficacy Endpoints
First Comparison of Amulet and Watchman FLX LAA Closure Devices Found Similar Outcomes


 July 31, 2024
July 31, 2024 









