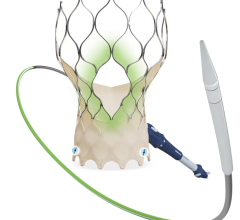
Abbott TriClip G4 device
May 20, 2022 — Abbott announced two late-breaking data presentations highlighting both TriClip, a first-of-its kind minimally invasive tricuspid heart valve repair device, and Navitor, the company's latest-generation transcatheter aortic valve implantation (TAVI) system. The company also showcased new data for MitraClip and Amplatzer Amulet, two key components of the company's industry-leading structural heart portfolio.
All data were presented at EuroPCR, the annual meeting of the European Association of Percutaneous Cardiovascular Interventions, held in Paris from May 17-20, 2022.
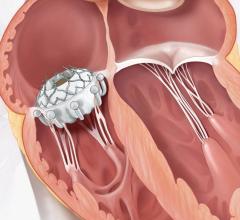
30-Day Results from the TriClip bRIGHT Study
New real-world outcomes highlighted in a late-breaking data presentation showed that the TriClip and TriClip G4 transcatheter edge-to-edge repair (TEER) systems significantly reduce tricuspid regurgitation (TR) and substantially improve quality of life across a wide range of anatomically diverse patients, with data through 30 days showing:
• High implant success rate (98%)
• Significant TR reduction (71% moderate or less compared to 3% as baseline) with a strong safety profile (99% freedom from major adverse events)
• Life-changing clinical improvements including 78% of patients achieving New York Heart Association (NYHA) Functional Class I/II (a classification of functional limitations resulting from cardiac disease), an improvement by 57% from baseline of 21%, and an 18-point improvement in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score (a self-assessment of social abilities, symptoms and quality of life)
"Historically, people suffering from severe tricuspid regurgitation had extremely limited treatment options despite being very ill. Many were ineligible for surgery and were limited to symptom management," said Philipp Lurz, M.D., Ph.D., professor and deputy head of cardiology, Heart Center Leipzig at University of Leipzig, Leipzig, Germany. "The late-breaking data show a high rate of implant success and significant reduction in regurgitation. Transcatheter edge-to-edge repair using TriClip has a huge potential to improve patients' quality of life and has entered the clinical stage with widespread use in Europe."
One-Year Results from Study on the Next-Generation Navitor TAVI System
Data from the multi-center, international, single arm study of the Navitor TAVI system with an active sealing cuff to minimize paravalvular leak (PVL) demonstrated improved one-year outcomes for patients with severe, symptomatic aortic stenosis who were at high or extreme surgical risk. Key findings include:
• High procedural success rate of 97.5%
• High rate of no/trace PVL (70.2%) and low rate of mild PVL (28.8%) through one year, indicating the active sealing cuff is effective in mitigating PVL
• Low rate of all-cause mortality (4.2%) at one year
• Single-digit gradients (average 7.5 mmHg) through one year
"Patients with symptomatic, severe aortic stenosis are often at high risk of complications from open-heart surgery due to their old age, frailty or having multiple other diseases and conditions," said Dave Smith, M.D, professor and consultant cardiologist, Morriston Hospital, Swansea, Wales. "The one-year results from the study demonstrate that a minimally invasive TAVI procedure with a Navitor valve offers a safe and effective treatment option for these patients."
Other data sets presented at EuroPCR included positive findings from the EXPAND study which showed MitraClip therapy in heart failure patients with mitral regurgitation experience improved symptoms and quality of life. Additionally, although women have higher rates of early complications with LAA closure than men, results from the Amulet IDE trial found that both women and men implanted with Abbott's Amplatzer Amulet Left Atrial Appendage (LAA) Occluder experienced similar long-term benefits from LAA closure.
"At Abbott, we're dedicated to delivering innovative technologies to help people with debilitating heart conditions live better lives through better health," said Michael Dale, senior vice president of Abbott's structural heart business. "The data presented during this year's EuroPCR meeting underscore our unwavering commitment to providing structural heart solutions, supported by clinical evidence, that surpass existing standards of care."
The TriClip Transcatheter Tricuspid Valve Repair System and Navitor Transcatheter Aortic Valve are approved for investigational use only in the U.S.
For U.S. important safety information on MitraClip: http://abbo.tt/MitraClipG4ISI
For U.S. important safety information on the Amplatzer Amulet LAA Occluder: https://abbo.tt/AmuletISI
Related MitraClip, Triclip, Amulet and Portico Content:
MitraClip Reduces Mortality for Heart Failure Patients With Secondary Mitral Regurgitation
VIDEO: MitraClip to Treat Heart Failure - Results of the COAPT Trial — Interview with William Abraham, M.D.,
VIDEO: Echocardiographic Findings in the COAPT Trial — Interview with Federico Asch, M.D.
FDA Approves MitraClip for Use in Heart Failure Patients With Functional Mitral Regurgitation
VIDEO: Impact of the COAPT Trial on Heart Failure Patients With Functional Mitral Regurgitation — Interview with Andreas Brieke, M.D.
Transcatheter Mitral Valve Repair is Cost-Effective in Heart Failure Patients
TAVR Expected to See Rapid Growth in Next 5 years
FDA Clears Abbott Amplatzer Amulet LAA Occluder to Reduce Stroke in People With Atrial Fibrillation
Portico TAVR System Found Safe and Effective for High-Risk Surgical Patients
Portico TAVR System Reduces Severe Aortic Stenosis at 30 Days in Real-World Setting


 July 08, 2024
July 08, 2024