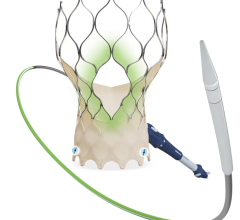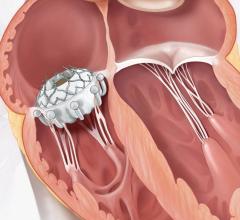
March 31, 2022 – Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel closure, today announced that the first patient was treated in a U.S. early feasibility study evaluating PerQseal+, the next generation of the company’s PerQseal device. The study is designed to evaluate PerQseal+ in percutaneous transcatheter aortic valve replacement (TAVR) procedures. PerQseal+ is intended to provide physicians with an even more robust solution for managing challenges and bleeding complications associated with large-bore arterial vessel closure.
The patient was treated at Stony Brook University Hospital in Stony Brook, New York by Robert Pyo, M.D., director of interventional cardiology and co-director of structural heart cardiology, Stony Brook Medicine.
“We are pleased to participate in this study of the PerQseal+ vessel closure device, a promising tool intended to advance the standard of care for patients undergoing minimally invasive structural heart procedures,” said William Gray, M.D., system chief, division of cardiovascular disease at Main Line Health, co-director at the Lankenau Heart Institute in Philadelphia and the principal investigator for the study. “This study will allow us to evaluate PerQseal+ for safe, efficient and secure large-bore vessel closure with a novel non-suture mechanism design, which has great potential for effectively managing vascular access without the complications typically associated with large-bore closure.”
Vivasure Medical’s first generation PerQseal device is the first sutureless and fully absorbable synthetic implant for large-bore arterial vessel punctures and is available to physicians in Europe. The novel PerQseal technology consists of an intravascular patch that seals the vessel from the inside, returning the artery to its natural state and does not leave the remains of any collagen, metal implants or sutures. The PerQseal+ device has an enhanced bioabsorbable patch designed to address more complex patient anatomies and is currently under clinical evaluation in Europe.
“Despite years of advancement, vascular complications remain the most common event for TAVR procedures, so further innovation is needed. Enrolling the first patient in this study is an important step forward in bringing a new, cutting-edge option for large-bore vessel closure to physicians in the U.S.,” said Andrew Glass, chief executive officer of Vivasure Medical. “We are looking to initiate a pivotal trial in the U.S. later this year to further evaluate our PerQseal technology for safety and efficacy in percutaneous large-bore endovascular procedures, which will support our submission to the FDA.”
Vivasure Medical is also developing PerQseal Blue, which is designed exclusively for sutureless and fully absorbable large-bore venous vessel closure following percutaneous cardiovascular procedures, such transcatheter mitral valve repair or replacement (TMVR) and transcatheter tricuspid valve repair or replacement (TTVR). Currently, there are no sutureless options for venous closure following large-bore venous closure procedures.
For more information: www.vivasuremedical.com


 July 08, 2024
July 08, 2024