PocketECG
February 14, 2022 – MediLynx Cardiac Monitoring, LLC (dba MediLynx Arrhythmia Diagnostics), a national remote monitoring provider and technology leader in deep learning artificial intelligence, has announced a partnership with the National Heart, Lung, and Blood Institute (NHLBI)/National Institutes of Health (NIH)-funded Cardiothoracic Surgical Trials Network (CTSN) to participate in a multicenter randomized clinical trial substudy of its PocketECG technology.
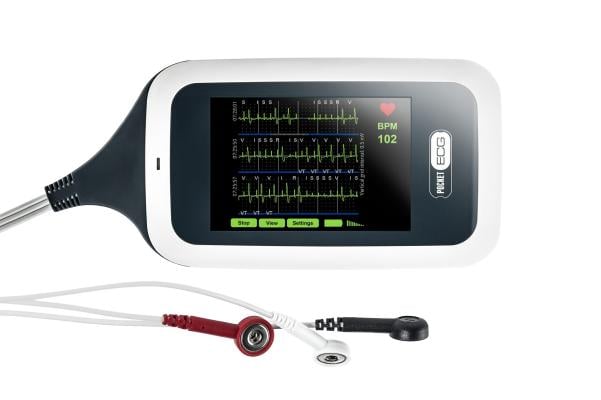
MediLynx’s PocketECG 4 combines advances in mobile data transmission, artificial intelligence, and cloud computing, in a novel device that provides for monitoring of continuous ECG for patients. The technology allows for streaming of every heartbeat to a cloud-based platform where the continuous signal is processed in near real-time by a Convolutional Neural Network with a super-deep structure of over 100 layers. ECG streaming combined with advanced cloud computing is ideally suited to accurately measure and predict Post-Operative Atrial Fibrillation (POAF).
The objective of the Anticoagulation for New-Onset Post-Operative Atrial Fibrillation after CABG (PACeS) Digital Health Substudy is to quantify the burden of POAF after isolated Coronary Artery Bypass Graft (CABG) surgery among enrolled patients.
The project will involve monitoring post-CABG patients for 30 days to determine the incidence of Atrial Fibrillation (AF). This is particularly important as post surgery AF can increase patient mortality. The study will involve the monitoring of 500 patients over a period of approximately four years.
Continuous Mobile Cardiac Telemetry (MCT) will be used to monitor patient heart rhythm for 30 days after hospital discharge. The secondary aim of this substudy will be to perform an exploratory analysis using MCT data that characterizes patterns of digital phenotyping for recurrent AF among POAF patients. Specifically, continuous MCT data will be used to explore three aspects of digital phenotyping: (1) actigraphy, (2) heart rate variability, and (3) micro-AF and Premature Atrial Contractions (PACs).
“The key to effective care management is identifying better solutions through research,” said William Moody, Chief Operating Officer at MediLynx. “This novel technology can properly assist physicians in identifying arrhythmias in patients, and accurately help determine the burden of such occurrence. MediLynx is honored to be selected by the NHLBI/NIH-funded CTSN to collaborate with more than 25 academic institutions on this exciting research study. MediLynx’s PocketECG solution was selected because of our modern engineering and proven clinical efficacy in cardiac monitoring.”
About the Trial
The PACeS Digital Health Substudy is a 500 patient study of a multicenter randomized clinical trial and will be conducted in highly experienced clinical centers participating in the CTSN. The estimated enrollment period is 48 months, and all participants will be provided with PocketECG devices for 30 days from the date of discharge.
For more information: https://medilynx.com/
Related Wearable ECG Monitoring Content:
How Advances in Wearable Cardiac Monitors Improve the Patient and Clinician Experience
Cardea Solo Wearable ECG Collects High-altitude Cardiac Data on Denali Expedition
Cardiac Insight Partners With VivoSense for Cardiovascular Research
Wearable Cardiac Monitors Are Effective for Tracking Atrial Fibrillation Following Ablation
As Interpretation Criteria Evolve, False Positive Athlete ECG Screening Rates Can Decrease

 July 24, 2024
July 24, 2024