A small number of patients develop myocarditis after receiving the messenger RNA (mRNA) COVID-19 vaccines from Pfizer and Moderna. This recently raised concerns after numbers for this adverse reaction jumped in April and May, prompting review this week by the Centers for Disease Control and Prevention (CDC) Advisory Committee on the Immunization Practices (ACIP). Getty Images
A small number of patients develop inflammation leading to myocarditis after receiving the messenger RNA (mRNA) COVID-19 vaccines from Pfizer and Moderna. This recently raised concerns after numbers for this adverse reaction to the vaccine jumped in April and May, prompting review this week at a meeting of the Centers for Disease Control and Prevention (CDC) Advisory Committee on the Immunization Practices (ACIP).
The CDC recommended the U.S. Food and Drug Administration (FDA) add a warning on the Moderna and Pfizer mRNA vaccine fact sheets about myocarditis being a rare, but possible side effect. The agency also said the side effect is very rare and patients generally recover quickly, The CDC said the risk of COVID is high and potentially more serious, so the CDC is continuing to encourage people to get the vaccine.
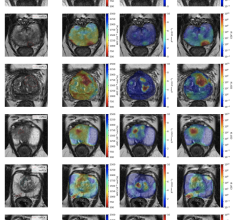
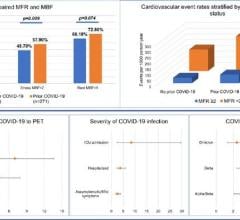
Between December 2020 and May 2021, there were 148 U.S. myocarditis cases reported from around the time a patient received a COVID-19 vaccination. But since April 2021, around the time most states opened up vaccinations to the general population and large numbers of younger people were vaccinated, the CDC said there has been a rapid increased in the reports of myocarditis to the CDC Vaccine Adverse Event Reporting System (VAERS) system. There are now 1,226 cases of myocarditis/pericarditis recorded in the VAERS data that are possibly related to COVID vaccines as of June 11, 2021. This is out of more than 318 million doses administered so far.
Vaccine statistical models created based on the trials of the mRNA vaccines predicted some cases of myocarditis, but the CDC data presented at the meeting this week showed numbers a quarter or third higher in younger age groups than what was expected.
These patients were mostly men aged 16-19, and symptoms usually occurred after the second dose, explained Tom Shimabukuro, M.D., MPH, MBA, deputy director of the CDC Immunization Safety Office, and a member of the Vaccine Safety Team, CDC COVID-19 Vaccine Task Force, at the ACIP meeting. He said most of these patients were hospitalized, but 95 percent of the cases were considered mild.
While there were cases of myocarditis in all age groups between 12-50, the majority of cases are concentrated in the 16-28 age group.
Of the 484 patients age 29 or younger, 323 met the CDC working case definition for myocarditis, pericarditis or both, Shimabukuro said. At the time of the report, 148 additional cases were still under review. Of the 323 patients, 309 were hospitalized. As of June 11, 295 patients had been discharged, nine were still in the hospital, including two in the ICU, and 14 were not hospitalized and were seen in the emergency department, urgent care or an outpatient clinic. Find more information in the CDC figures after the end of the article below.

CDC VAERS reports of myocarditis related to the COVID-19 vaccines presented at the CDC ACIP meeting Tuesday.
The CDC said nearly all follow-up visit notes for these patients indicated resolution of symptoms at the time of follow-up. Of those that had follow-up ECG, echo and lab testing, most had returned to normal or baseline levels.
The vast majority of patients sought medical attention for myocarditis after their second dose. Of these, about 80 cases reported symptoms starting on the day they were vaccinated. Most cases (more than 540) reported symptoms within four days of receiving the vaccine.
The CDC reported the following stats on these myocarditis cases:
• Median age of patients was 30 for dose 1 reports and 24 for dose 2 reports.
• Median time to symptom onset was 3-4 days
• Males were affected more than females, 66 vs. 33 percent, in dose 1 patients, and 79 vs. 20 percent in dose 2 reports.
Symptoms reported include:
• Chest pain (416 patients)
• Elevated cardiac enzymes (310 patients)
• ST or T wave changes on ECG (295 patients)
• Dyspnea (117 patients)
• Abnormal echocardiography (81 patients)

CDC report on VAERS data on possible COVID vaccine caused myocarditis cases broken down by age.
FDA Adds Myocarditis Warning to Vaccine Clinician Fact Sheets
In response to the CDC committee review, the FDA announced revisions June 25 to the patient and provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines. The FDA said these revisions include the suggestion the vaccines may cause increased risks of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the tissue surrounding the heart) following vaccination.
For each vaccine, the Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) has been revised to include a warning about myocarditis and pericarditis and the Fact Sheet for Recipients and Caregivers has been revised to include information about myocarditis and pericarditis.
The FDA said this update follows an extensive review of information and the discussion by CDC’s Advisory Committee on Immunization Practices meeting June 23. The data presented at this meeting reinforced the FDA’s decision to revise the fact sheets and further informed the specific revisions.
The warning in the Fact Sheets for Healthcare Providers Administering Vaccines notes that reports of adverse events suggest increased risks of myocarditis and pericarditis, particularly following the second dose and with onset of symptoms within a few days after vaccination. Additionally, the Fact Sheets for Recipients and Caregivers for these vaccines note that vaccine recipients should seek medical attention right away if they have chest pain, shortness of breath, or feelings of having a fast-beating, fluttering or pounding heart after vaccination. The FDA and CDC are monitoring the reports, collecting more information, and will follow-up to assess longer-term outcomes over several months.
Link to the revised Moderna COVID-19 Vaccine Fact Sheet
Link to the revised Pfizer-BioNTech COVID-19 Vaccine fact sheet
Israel Was First to Identify the Link with Myocarditis Cases and the COVID Vaccine
Israel's vaccination program moved very quickly early on and was the first to determine there was a possible link with the vaccine and the onset of myocarditis in young men ages 16-30. The first reported case of myocarditis was a 19-year-old who was hospitalized Feb 1.[1] However, they determined there was not a significant safety issue after reviewing the case.[2]
Israel determined the risks of complications from coronavirus outweighs the risks posed by the vaccine's side effects, explained Matthew Daley, M.D., CDC Advisory Committee on Immunization Practices work group chair, and a pediatrician with Colorado Permanente Medical Group. He explained at the meeting the Israelis noted that the myocarditis cases, mainly observed in teenagers 16-19, were very low and in most cases passed with no complications. For these reasons, he said Israel pushed ahead with its nation-wide vaccination efforts.
CDC Reiterates the Vaccines Are Safe
Like Israel, the CDC is still advocating for everyone who can to be vaccinated. The CDC said the cases of myocarditis in teens and young adults is rare, and the risks of COVID outweigh the risks of these rare side effects.
The CDC reported a myocarditis rate of 12.6 cases per million after the administration of the second dose of the vaccine in the 21 days following vaccination. The rate is higher in males than females. The available outcome data indicate that patients generally recover from symptoms and do well. The CDC reports that in most cases, patients who presented for medical care responded well to medication and rest with prompt improvements of symptoms.
The CDC said it will continue to monitor VAERS reports and VSD data by following up on myocarditis cases by obtaining medical records, conduct case reviews and apply the CDC working definition to adjudicate case reports.
The CDC is interested in longer-term effects, so it plans to gather outcome data for these patients at 3 and 6 months.
The reports of elevated numbers of myocarditis in younger vaccinated patients was mainly seen in the mRNA vaccines. While some cases have been reported cases with the Janssen (Johnson and Johnson) vaccine, there is no clear trend as has been seen with the mRNA vaccines.
CDC Recommendations for Clinicians
The CDC has set up a specific website page to distribute information on the vaccine-caused myocarditis at www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html. It includes these recommendations for clinicians:
• CDC continues to recommend COVID-19 vaccination for everyone 12 years of age and older given the greater risk of other serious complications related to COVID-19, such as hospitalization, multisystem inflammatory syndrome in children (MIS-C), or death.
• Report all cases of myocarditis and pericarditis post COVID-19 vaccination to VAERS.
• Consider myocarditis and pericarditis in adolescents or young adults with acute chest pain, shortness of breath, or palpitations. In this younger population, coronary events are less likely to be a source of these symptoms.
• Ask about prior COVID-19 vaccination if you identify these symptoms, as well as relevant other medical, travel and social history.
• For initial evaluation, consider an ECG, troponin level, and inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate. In the setting of normal ECG, troponin, and inflammatory markers, myocarditis or pericarditis are unlikely.
• For suspected cases, consider consultation with cardiology for assistance with cardiac evaluation and management. Evaluation and management may vary depending on the patient age, clinical presentation, potential causes or practice preference of the provider.
• For follow-up of patients with myocarditis, consult the recommendations from the American Heart Association and the American College of Cardiology.
• It is important to rule out other potential causes of myocarditis and pericarditis. Consider consultation with infectious disease and/or rheumatology to assist in this evaluation.
• Where available, evaluate for potential etiologies of myocarditis and pericarditis, particularly acute COVID-19 infection (e.g., PCR testing), prior SARS-CoV-2 infection (e.g., detection of SARS-CoV-2 nucleocapsid antibodies), and other viral etiologies (e.g., enterovirus PCR and comprehensive respiratory viral pathogen testing).
Myocarditis Causes, Incidence and Treatment
Traditional causes of myocarditis include viral, bacterial, parasitic and fungal infections, toxic such as anthracycline cancer drugs or cocaine, hypersensitivity to various drugs such as diuretics, dobutamine and tricyclic antidepressants, or from immunological syndromes such as diabetes, inflammatory bowel syndrome, sarcoidosis and others, explained Matthew Oster, M.D., MPH, CDC COVID-19 Vaccine Task Force and a pediatric cardiologist at Children's Healthcare of Atlanta, at the ACIP meeting.
The fact that myocarditis disproportionally affects males is not surprising. Oster said studies show the annual average incidence of myocarditis show males are disproportionally effected. In patients ages 15-18, he said the incidence is about 1.8 per 100,000 people, 66% of patients are male and the median length of stay is 6.1 days.[3] For adults, the incidence of myocarditis gradually decreases with age, and most patients with the condition (76%) are male.[4]
He said treatment of myocarditis mainly involves supportive care. But, it also can include directed care or arrhythmias, decreased heart function and congestive heart failure. The role of anti-inflammatory medicines is unclear. There also is a recommendation of restricting exercise while the heart recovers.[5]
Overview of mRNA Coronavirus Vaccine Myocarditis Cases in Medical Literature
Here is a list of some publications on this issue from medical literature:
• Marshall et al – 7 healthy males 14-19 within 4 days of 2nd mRNA vaccine.[6]
Five patients had fever around the time of presentation. Acute COVID-19 was ruled out in all 7 cases based on negative PCR tests. Six of the 7 patients had negative SARSCoV-2 nucleocapsid antibody assays, suggesting no prior infection. All patients had an elevated troponin. Cardiac magnetic resonance imaging (MRI) revealed late gadolinium enhancement characteristic of myocarditis. All 7 patients resolved their symptoms rapidly. Three patients were treated with non-steroidal anti-inflammatory drugs (NSAIDs) only and 4 received intravenous immune globulin (IVIG) and corticosteroids. All patients were discharged home after 2-6 days in the hospital (median 4 days).
• Rosner et al – 5 males 19-39 within 4 days of 2nd dose of vaccine, 1 24-year-old male 7 days after 1st dose.[7]
Study included 7 patients hospitalized for acute myocarditis-like illness following a COVID-19 vaccination, treated at hospitals in Falls Church, Va., and Dallas, Texas. All patients were males under the age of 40. The youngest was 19 and the oldest was 39. Six were white, one was Hispanic. Only one patient reported a history of previous COVID-19 infection. Six patients were tested negative. Six received mRNA COVID-19 vaccines by Pfizer/BioNTech and one by Moderna. One patient received the adenovirus Johnson & Johnson COVID-19 vaccine. All patients were hospitalized within 3-7 days after receiving a vaccine with sudden onset chest pain. Myocardial injury was confirmed by either cardiac troponin I or elevated high sensitivity troponin testing. All patients had stable vital signs. ECG results varied from a normal heart rhythm to ST-segment elevation, which can indicate a decrease in blood flow to the heart muscle. The three patients who underwent invasive coronary angiography showed no signs of coronary blockage. None of the patients reported heart palpitations, and none had signs of heart arrhythmias. Treatment varied and included beta-blocker and anti-inflammatory medications. All symptoms were resolved before hospital discharge.
• Larson et al – 8 males 22-56 hospitalized After receive COVID vaccines.[8]
This study included 4 U.S. and 4 Italian patients. Seven patients had onset of syptoms within four days of dose 2, one had onset 2 days after dose 1 and had a prior SARS-CoV-2 infection. All had abnormal troponin, echo and MRI findings, and seven had abnormal ECG. Four patients were treated with NSAIDs or colchicine, steroids in two, no treatment in three. All were discharged home with resolution of symptoms and preserved ejection fraction.
• The U.S. military identified 14 mayocarditis cases following mRNA vaccination.[9]
The U.S. Defense Department said in April it was tracking 14 cases of myocarditis in military health patients after receiving either the Pfizer or Moderna COVID-19 vaccine. All patients experienced chest pain 12 to 96 hours after vaccination. One was hospitalized at Walter Reed National Military Medical Center in February and spent nearly three days in the ICU, but returned to work with the National Guard in Washington, D.C. Another 39-year-old male in the Washington D.C. area spent two days in the hospital after his second Pfizer dose. Discharge instructions included no PT, exercise, marching or drill for the next six months. Although most patients were young and fit, military clinicians drew on experiences with the ACAM2000 smallpox vaccine in 2003, and referred the patients for cardiac evaluations in which the myocarditis was diagnosed. The 2003 smallpox vaccine caused at least 10 military personnel and several civilians to develop myocarditis after receiving the vaccine. Two died of heart attacks and the CDC took steps to recommend that people with known heart disease avoid the smallpox vaccination.
• Israeli Ministry of Health - 148 myocarditis cases occurring within 30 days of mRNA
vaccine.[2]
• 27 cases out of ~5.4 million first doses
• 121 cases out of ~5 million second doses
• Mostly in men aged 16-30 (particularly 16-19)
• Most were in the hospital up to 4 days
• 95% of cases considered mild
Related Content on the Side Effects of the COVID Vaccines:
Small Number of Patients Have Myocarditis-like Illness After COVID-19 Vaccination
CDC and FDA Call for Pause on Janssen COVID-19 Vaccine Due to Rare Blood Clots
VIDEO: COVID Vaccine Adenopathy Can Last Up to 10 Weeks
COVID-19 Vaccine Can Cause False Positive Cancer Diagnosis
VIDEO: COVID Vaccine May Cause Enlarged Lymph Nodes on Mammograms
Find more cardiology related COVID content

CDC VAERS statistics on COVID vaccine caused myocarditis cases by age, sex and symptom onset as on June 2021.

CDC myocarditis working definition being used in reviewing possible COVID vaccine caused myocarditis cases.

CDC pericarditis working definition being used in reviewing possible COVID vaccine caused myocarditis cases.
References:
5. Leslie T. Cooper. Myocarditis. N Engl J Med 2009; 360:1526-1538. DOI: 10.1056/NEJMra0800028

 March 20, 2024
March 20, 2024