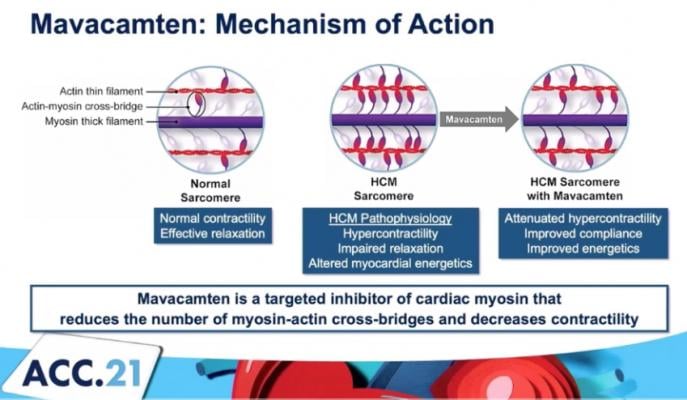
The mechanism of action for mavacamten, a cardiac myosin inhibitor to treat obstructive hypertrophic cardiomyopathy (oHCM).
May 15, 2021 — A new analysis of the Phase 3 EXPLORER-HCM study evaluating mavacamten, an investigational, first-in-class cardiac myosin inhibitor, in patients with obstructive hypertrophic cardiomyopathy (oHCM), found at 30 weeks improvement in the Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ OSS) was greater in mavacamten patients than placebo.
The late-breaking data was presented at the American College of Cardiology (ACC) 2021 scientific session, with simultaneous publication in The Lancet.[1]
“The KCCQ is a 23-item disease-specific questionnaire quantifying symptoms, physical function, social function and quality of life. By using this tool, we were able to demonstrate substantial clinical benefits for patients taking mavacamten in the trial, which diminished when patients ended treatment,” said lead study investigator, John A. Spertus, M.D., M.P.H., clinical director of outcomes research at Saint Luke’s Mid America Heart Institute and the Lauer Missouri Endowed Chair and Professor of Medicine at the University of Missouri–Kansas City. “This new analysis of the EXPLORER-HCM data provides important insights into the benefits myosin inhibition can have in improving the health status of patients with severe obstructive hypertrophic cardiomyopathy, a chronic, often debilitating condition.”
Bristol Myers Squibb, the maker of the drug, reported there were similar benefits across all KCCQ subscales. Moreover, a greater proportion of mavacamten patients achieved a very large, clinically meaningful improvement (≥20 points) in the KCCQ OSS, compared to placebo, 36% [33/92] vs. 15% [13/88]. A change of at least 5 points is required to be considered clinically significant.
In the Phase 3, double-blind, placebo-controlled trial, patients with symptomatic oHCM (LVOT gradient ≥50 mmHg and NYHA Class II-III) were randomized 1:1 to mavacamten (n=123) or placebo (n=128) for 30 weeks, followed by an 8-week washout. The KCCQ was administered at baseline and Weeks 6, 12, 18, 30 and 38. Change from baseline in KCCQ scores were analyzed using mixed model repeated measures and responder analyses.
A total of 92 patients randomized to mavacamten and 88 randomized to placebo completed the KCCQ at both baseline and Week 30 (end of treatment).
Key Findings of the EXPLORER-HCM Study
At 30 weeks, the change in KCCQ OSS was greater with mavacamten than placebo (mean ± SD, 14·9±16 vs. 5·4±14; difference=9·1 (95%CI: 5·5-12·8; p<0·001), with similar benefits across all KCCQ subscales.
The proportion of patients with a very large change (KCCQ OSS ≥20 points) was 36% [33/92] vs. 15% [13/88], with an estimated absolute difference of 21% (95% CI=8·8%, 33·4%) and number needed to treat of 5 (95% CI=3, 11). A change of at least 5 points is considered clinically important. These gains returned to baseline after active treatment was stopped.
A greater proportion of patients in the placebo arm had no change or deterioration in their health status at Week 30.
“Mavacamten represents Bristol Myers Squibb’s ongoing dedication to improving the lives of patients, especially those living with chronic cardiovascular diseases such as oHCM, through scientific discovery,” said Jay Edelberg, M.D., Ph.D., head, Heart Failure and Cardiomyopathy Development at Bristol Myers Squibb. “This new analysis of data from the Phase 3 EXPLORER-HCM trial further supports the scientific evidence suggesting the benefit mavacamten can have on improving health status, symptoms and quality of life in patients with symptomatic oHCM and we look forward to potentially bringing this important new therapy to patients next year.”
About the Phase 3 EXPLORER-HCM Mavacamten Hypertrophic Cardiomyopathy Trial
The EXPLORER-HCM Phase 3 trial enrolled a total of 251 patients with symptomatic (NYHA Class II or III), obstructive hypertrophic cardiomyopathy. All participants had measurable left ventricular outflow tract (LVOT) gradient (resting and/or provoked) ≥50 mmHg at baseline.
The primary endpoint for EXPLORER-HCM was a composite functional analysis designed to capture mavacamten’s effect on both symptoms and function. Secondary endpoints were changes from baseline to week 30 in post-exercise LVOT gradient, pVO2, proportion of patients with at least one NYHA class improvement, and measures of patient reported outcomes. Additional endpoints included changes from baseline to Week 30 in echocardiographic indices, circulating biomarkers, cardiac rhythm patterns and accelerometry.
About Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy, or HCM, is a chronic, progressive disease in which excessive contraction of the heart muscle and reduced ability of the left ventricle to fill can lead to the development of debilitating symptoms and cardiac dysfunction. In HCM patients, exertion can result in fatigue or shortness of breath, interfering with a patient’s ability to participate in activities of daily living. Furthermore, HCM has also been associated with increased risks of atrial fibrillation, stroke, heart failure and sudden cardiac death. The most frequent cause of HCM is mutations in the heart muscle proteins of the sarcomere. HCM is estimated to affect one in every 500 people, however many patients remain undiagnosed and/or asymptomatic.
Mavacamten is a Cardiac Myosin Inhibitor
Mavacamten is a potential first-in-class, oral, allosteric modulator of cardiac myosin, under investigation for the treatment of conditions in which excessive cardiac contractility and impaired diastolic filling of the heart are the underlying cause. Mavacamten reduces cardiac muscle contractility by inhibiting excessive myosin-actin cross-bridge formation that results in hypercontractility, left ventricular hypertrophy and reduced compliance. In clinical and preclinical studies, mavacamten has consistently reduced biomarkers of cardiac wall stress, lessened excessive cardiac contractility and increased diastolic compliance.
For more information: BMS.com
Find links to more ACC 2021 late-breakers
VIDEO: Septal Ablation to Treat Hypertrophic Cardiomyopathy — Interview with Carey Kimmelstiel, M.D.
Reference:


 July 31, 2024
July 31, 2024 









