An ECMO procedure at Tufts Medical Center in Boston. The FDA cleared the use of ECMO support for COVID-19 patients in April 2020. A new review of patients treated with ECMO showed it had positive outcomes in the most severe COVID-19 patients. Photo by Dave Fornell
September 29, 2020 – Extracorporeal membrane oxygenation (ECMO) support saved lives in past epidemics of lung-damaging viruses and it is now doing the same for many of the critically ill COVID-19 (SARS-CoV-2) patients who receive it, according to a new international study in The Lancet.[1]
The new study provides strong support for the use of ECMO in appropriate patients as the pandemic rages on worldwide. The 1,035 patients in the study faced a staggeringly high risk of death, as ventilators and other care failed to support their lungs. But after they were placed on ECMO, their actual death rate was less than 40%. That is similar to the rate for patients treated with ECMO in past outbreaks of lung-damaging viruses, and other severe forms of viral pneumonia.
“These results from hospitals experienced in providing ECMO are similar to past reports of ECMO-supported patients, with other forms of acute respiratory distress syndrome or viral pneumonia,” said co-lead author Ryan Barbaro, M.D., of Michigan Medicine, the University of Michigan’s academic medical center. “These results support recommendations to consider ECMO in COVID-19 if the ventilator is failing. We hope these findings help hospitals make decisions about this resource-intensive option.”
The new data may help more hospitals that have ECMO capability understand which of their COVID-19 patients might benefit from the technique, which channels blood out of the body and into a circuit of equipment that adds oxygen directly to the blood before pumping it back into regular circulation. Small studies published early in the pandemic had cast doubt on the technique’s usefulness.
Still, the international team of authors cautions that patients who show signs of needing advanced life support should receive it at hospitals with experienced ECMO teams, and that hospitals should not try to add ECMO capability mid-pandemic.
Watch the VIDEO: ECMO Support Effective in Sickest COVID-19 Patients — Interview with Ryan Barbaro, M.D.
Global Cooperation Achieved Fast Clinical Data on ECMO for COVID Patients
The study was made possible by a rapidly created international registry that has given critical care experts near real-time data on the use of ECMO in COVID-19 patients since early in the year.
Hosted by the Extracorporeal Life Support Organization (ELSO), the registry includes data submitted by the 213 hospitals across 36 countries on four continents whose patients were included in the new analysis. The paper includes data on patients age 16 or older who were started on ECMO between January 16 and May 1, and follows them until death, discharge from the hospital, or August 5, whichever occurred first. The team will present the findings at the ELSO Annual Meeting on Sept. 26.
“At the beginning of the COVID-19 pandemic, the role of ECMO was unknown. In response the international ELSO community began collecting and sharing real-time high-quality data through the ELSO Registry.” said Barbaro, who is also chair of ELSO’s Registry Committee. “The results of this study are a witness of those efforts and the findings support recommendations to consider ECMO when lung protective ventilation fails."
The generalizable estimate of ECMO mortality and improved mortality rate findings support existing recommendations to consider ECMO in refractory COVID-19-related respiratory failure when performed in experienced centers.
Co-lead author Graeme MacLaren, MBBS, of the National University Health System in Singapore, notes, “Most centers in this study did not need to use ECMO for COVID-19 very often. By bringing data from over 200 international centers together into the same study, ELSO has deepened our knowledge about the use of ECMO for COVID-19 in a way that would be impossible for individual centers to learn on their own.”
Insights Into Outcomes of COVID Patient Treated With ECMO
Seventy percent of the patients in the study were transferred to the hospital where they received ECMO. Half of these were actually started on ECMO – likely by the receiving hospital’s team -- before they were transferred. This reinforces the importance of communication between ECMO-capable hospitals and non-ECMO hospitals that might have COVID-19 patients who could benefit from ECMO.
The new study could also help identify which patients will benefit most if they are placed on ECMO.
“Our findings also show that mortality risk rises significantly with patient age, and that those who are immunocompromised, have acute kidney injuries, worse ventilator outcomes or COVID-19-related cardiac arrests are less likely to survive,” said Barbaro, who chairs ELSO’s COVID-19 registry committee and provides ECMO care as a pediatric intensive care physician at University of Michigan’s C.S. Mott Children’s Hospital. “Those who need ECMO to replace cardiac function as well as lung function also did worse. All of this knowledge can help centers and families understand what patients might face if they are placed on ECMO.”
“The lack of reliable information early in the pandemic hampered our ability to understand the role of ECMO for COVID-19,” said co-senior author Daniel Brodie, M.D., of New York Presbyterian Hospital. “The results of this large-scale international registry study, while hardly definitive evidence, provide a real-world understanding of the potential for ECMO to save lives in a highly selected population of COVID-19 patients.” Brodie shares senior authorship with Roberto Lorusso, M.D. of the Maastricht University Medical Center in the Netherlands and Alain Combes, M.D. of Sorbonne University in Paris.
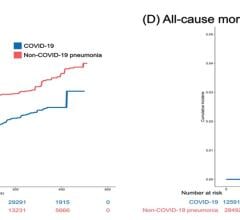
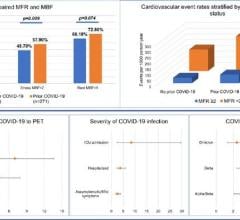
Among the 1,035 eligible ECMO-supported patients with COVID-19, with 67 (6 percent) remaining hospitalized at the end of the study period, the estimated cumulative incidence of in-hospital mortality 90 days after the initiation of ECMO was 37.4 percent. The mortality rate was 39 percent (380/968) among those with a final disposition of death or hospital discharge.
The results support current recommendations that centers experienced in ECMO should consider its use in refractory COVID-19-related respiratory failure.
A Robust Statistical Approach Used in COVID ECMO Study
Because the ELSO database does not track what happens to patients once they are discharged to home, other hospitals, and long-term acute care or rehabilitation facilities, the study used a statistical approach based on in-hospital mortality up to 90 days after the patient was put ECMO. This also allows the team to account for the 67 patients who were still in the hospital as of August 5, whether they were still on ECMO, in the ICU or in step-down units.
Philip Boonstra, Ph.D., of the University of Michigan School of Public Health, helped design the study using a “competing risk” approach, based on his experience handling the statistical design and analysis of long-term data from clinical trials for cancer.
“We used 90-day in-hospital mortality because this is the highest-risk period, and because it allows us to use the information we have to the fullest, even if we don’t know the final outcome for every patient,” he says.
Having data through August, when only a small number of the patients in the study remained in the hospital, was important – though data are missing on a small number of patients. And even though patients who were discharged to their homes or a rehabilitation facility will likely have a long recovery ahead after the intensive level of care involved in ECMO, they are likely to survive based on past data. However, the fate of those who went to LTAC facilities, which provide long-term care at a near-ICU level, is less certain.
Next Steps in Use of ECMO for Severe COVID Patients
More than half of the patients in the study were treated in hospitals in the United States and Canada, including Michigan Medicine’s own hospitals. University of Michigan's Robert Bartlett, M.D., emeritus professor of surgery and a co-author of the new paper, is considered a key figure in the development of ECMO, including the first use in adults in the 1980s. Bartlett led the development of the initial guidance for the use of ECMO in COVID-19.
“ECMO is the final step in the algorithm for managing life-threatening lung failure in advanced ICUs,” said Bartlett. “Now we know it is effective in COVID-19.”
As of August 5, 380 of the patients in the study had died in the hospital, more than 80% of them within 24 hours of a proactive decision to discontinue ECMO care because of a poor prognosis. Of the remaining patients 57% had gone home or to a rehabilitation center (311 patients); had been discharged to another hospital or a long-term acute care center (277 patients). The rest were still in the hospital but had reached 90 days after start of ECMO.
The new study adds to the information used to create the ECMO COVID-19 guidelines published by ELSO, which is in part based on past randomized controlled trials of ECMO’s use in acute respiratory syndrome (ARDS).
Barbaro and others are studying the longer-term effects of ECMO care for any patient; he leads a team that has recently received a National Institutes of Health (NIH) grant for a long-term study of children who have survived after treatment with ECMO.
Meanwhile, the ELSO registry continues to track the care of patients placed on ECMO because of COVID-19. Christine Stead, the chief executive officer of ELSO, credits the rapid pivot and intense teamwork among ECMO centers and their staff for the strength of the new paper.
“We started with a WeChat dialogue with teams in China, who were able to share knowledge and help their counterparts in Japan be ready for the spread to their country,” Stead explained. “We asked all the centers that take part in ELSO to change their practice, and begin entering data about patients as soon as they were placed on ECMO, rather than waiting until they were discharged from the hospital. This has allowed us to achieve something that will help hospitals make more informed decisions, based on meaningful data, as the pandemic continues.”
Use of ECMO in COVID-19 Patients
The U.S. Food and Drug Administration (FDA) in April 2020 expanded the availability of ECMO therapy to address the COVID-19 public health emergency. ECMO systems are used for long-term respiratory/cardiopulmonary failure and provide assisted extracorporeal circulation and physiologic gas exchange of the patient’s blood for more than six hours. The technology can oxygenate a severely sick COVID-19 pneumonia patient's blood without the need to transfer the oxygen through fluid filled lungs.
As a sudden acute respiratory syndrome, COVID-19 can trigger acute respiratory failure and/or acute cardiopulmonary failure. Under these conditions, the FDA states in the new policy extracorporeal oxygenation can be used for greater than six hours as tool for treating patients. ECMO is often used in COVID patients as a last resort, after mechanical ventilators fail to provide enough support for the patient.
About the Extracorporeal Life Support Organization (ELSO)
The Extracorporeal Life Support Organization (ELSO) is an international non-profit organization comprised of health care institutions and individuals dedicated to the development and evaluation of novel therapies for the support of failing organ systems. The organization’s mission is to provide continuing education, guideline development, original research, publications, and maintenance of a comprehensive registry of data around use of extracorporeal membrane oxygenation (ECMO) in active ELSO centers. Learn more at www.elso.org.
Watch the VIDEO: ECMO Support Effective in Sickest COVID-19 Patients — Interview with Ryan Barbaro, M.D.
Related COVID-19 ECMO Support Content:
FDA Approves ECMO to Treat COVID-19 Patients
VIDEO: ECMO Support Effective in Sickest COVID-19 Patients — Interview with Ryan Barbaro, M.D.
ECMO Used to Treat Adult Respiratory Distress Syndrome Case
ECMO Support Found Effective in Sickest of COVID-19 Patients
LivaNova Modifies its ECMO Indications Beyond Six Hours to Address COVID-19
FDA Allows Emergency Use of Impella for COVID-19 Patients on ECMO
The Cardiovascular Impact of COVID-19
VIDEO: Multiple Cardiovascular Presentations of COVID-19 in New York — Interview with Justin Fried, M.D., explaining a case that used VV-ECMO abnd VAV-ECMO
VIDEO: Impact of COVID-19 on the Interventional Cardiology Program at Henry Ford Hospital — Interview with William O'Neill, M.D.
Kawasaki-like Inflammatory Disease Affects Children With COVID-19
Find more cardiology related COVID-19 content
Reference:

 March 20, 2024
March 20, 2024