March 19, 2019 – iSchemaView announced the company’s co-founder Gregory Albers, M.D., has received the Distinguished Clinical Research Achievement Award, presented by the Clinical Research Forum. The Distinguished Clinical Research Achievement Awards are presented to the top two studies that show creativity, innovation, or a novel approach that demonstrates an immediate impact on the health and well-being of patients.
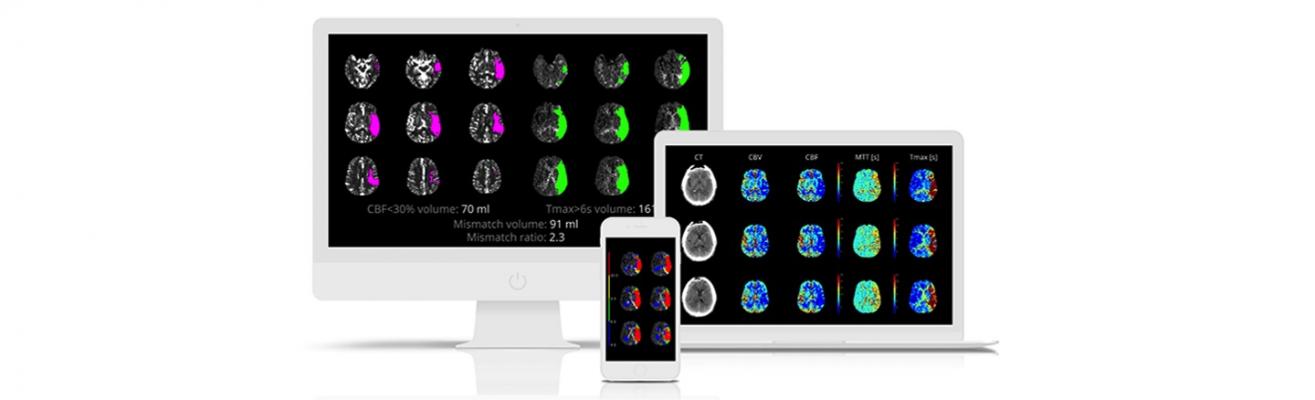
In the DEFUSE 3 study, Albers and his team used iSchemaView’s RAPID advanced medical imaging platform, exclusively, to identify stroke patients who continue to have salvageable brain tissue many hours after stroke onset. By identifying patients who are most likely to benefit from stroke therapies, the DEFUSE 3 study is credited with accelerating the development and testing of new therapeutic options, as well as enabling hospitals to offer life-saving stroke therapies to many patients who arrive after the previous standard six-hour treatment window has elapsed.
Albers is the Coyote Foundation professor of neurology and neurological sciences and professor, by courtesy, of neurosurgery at the Stanford University Medical Center. He is also director of the Stanford Stroke Center. Albers’ primary research focus is the diagnosis, management and prevention of ischemic stroke. He was instrumental in the development of RAPID, a medical imaging platform that allows physicians to identify stroke patients who have salvageable brain tissue. Due to the findings of DEFUSE 3 and other stroke studies that also employed RAPID, the American Heart Association and American Stroke Association changed their treatment guidelines for thrombectomy from six hours after onset to up to 24 hours after onset for patients with salvageable brain tissue.
Following the awards ceremony, Albers will present the results of the DEFUSE 3 study to members of Congress and discuss the critical role of federal funding for clinical research.
For more information: www.i-rapid.com


 February 02, 2026
February 02, 2026 









