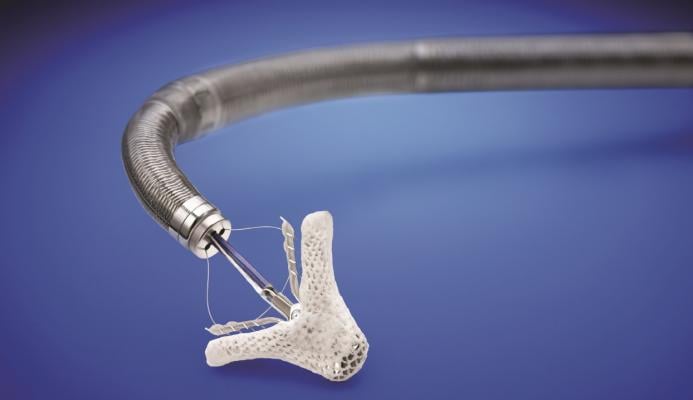
March 14, 2019 — The U.S. Food and Drug Administration (FDA) approved the MitraClip heart valve repair device for patients with heart failure symptoms and moderate-to-severe or severe mitral regurgitation (MR) due to diminished left heart function, commonly known as secondary or functional MR, despite optimal medical therapy. MitraClip was originally indicated for patients with significant MR and heart failure symptoms resulting from abnormalities of the mitral valve, commonly known as primary or degenerative MR.
The MitraClip, manufactured by Abbott Vascular, is intended to reduce moderate-to-severe or severe mitral regurgitation, a leakage of blood backward through the mitral valve into the heart’s left atrium that can cause heart failure symptoms such as shortness of breath, fatigue and swelling in the legs.
“Expanding the approval of this device to heart failure patients with significant secondary mitral regurgitation, who have failed to get symptom relief from other therapies, provides an important new treatment option,” said Bram D. Zuckerman, M.D, director of the Division of Cardiovascular Devices in the Center for Devices and Radiological Health. “Careful evaluation by a team of specialists is essential to determining whether a particular patient is an appropriate candidate for this procedure.”
About 6.5 million American adults live with heart failure, a chronic, progressive condition in which the heart muscle is unable to pump enough blood to meet the body’s needs for blood and oxygen. A small percentage of these patients also have moderate-to-severe or severe secondary MR, increasing the risks and complicating the treatment of their heart failure. With this new approval, this small percentage of patients could be indicated as candidates for treatment with the MitraClip device when combined with optimal medical therapy. Optimal medical therapy includes combinations of different heart failure medications along with, in certain patients, cardiac resynchronization therapy and implantation of cardioverter defibrillators.
The MitraClip is inserted in a minimally invasive procedure through the femoral vein in the leg and guided into the heart’s left ventricle where it grasps the two leaflets of the mitral valve, clipping them together to reduce the backflow of blood.
The approval of the new indication is based on the COAPT study of 614 patients, which represented a paradigm shift in how heart failure patients will be managed in the future. For years the focus on heart failure has been on the ventricles and secondary mitral regurgitation (MR) has been seen as a symptom of worsening heart failure, with little attention paid to fixing it. COAPT looked at patients with heart failure who had moderate-to-severe or severe secondary MR and were randomly assigned to get either continuation of their optimized medication treatment plus the MitraClip (MitraClip group) or continuation of their optimized medication treatment only (control group). The risk of being re-hospitalized for heart failure symptoms was reduced by approximately 47 percent in the MitraClip group compared to the control group. In addition, the risk of death within two years was decreased by approximately 37 percent in the MitraClip group compared to the control group.
Read the blog "Key Interventional Cardiology News and New Technology at TCT 2018"
The 2019 American College of Cardiology (ACC) annual scientific sessions, March 16-18 in New Orleans, will feature late-breaking presentations on several COAPT subanalyses, including:
- COAPT Echocardiographic Outcomes: Mitral Regurgitation After MitraClip Implantation in Patients with Heart Failure and Secondary Mitral Regurgitation; and
- COAPT Quality of Life After Transcatheter Mitral-Valve Repair in Patients with Heart Failure and Secondary Mitral Regurgitation. Results from the COAPT trial
"We now know fixing the valve matters. The MitraClip improves survival," said William Abraham, M.D., director of the Cardiovascular division, The Ohio State University, one of the principal investors in the COAPT trial. "I would consider this a landmark trial and a real game-changer for our patients. It is the first time we have ever seen in any randomized, controlled trial an outright reduction in heart failure hospitalizations, all-cause mortality, and improvements in quality of life and exercise to a tremendous magnitude."
Watch the VIDEO: MitraClip to Treat Heart Failure - Results of the COAPT Trial, an interview with Abraham at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference.
Watch the VIDEO: Impact of the COAPT Trial on Heart Failure Patients With Functional Mitral Regurgitation, an interview with Andreas Brieke, M.D., director of mechanical circulatory support, heart failure physician and site principal investigator for the COAPT Trial at the University of Colorado Hospital
Potential adverse events from the device and implant procedure include death, stroke, major bleeding, and erratic heart beat (atrial fibrillation).
The MitraClip is contraindicated in patients who cannot tolerate blood thinners during or after the procedure, who have active inflammation (endocarditis) of the mitral valve, rheumatic mitral valve disease or evidence of blood clots in the heart or veins leading to the heart.
Abbott said based on this approval, the company will begin discussions with the Center for Medicare and Medicaid Services (CMS) and physician specialty societies to request a revision to the national coverage determination (NCD) that would expand Medicare coverage to include secondary MR patients.
"Since severe secondary MR is extremely difficult to manage and associated with a poor prognosis, people have historically had few options," said Neil Moat, M.D., chief medical officer of Abbott's structural heart business. "The expanded indication of MitraClip opens new doors for these ailing patients and can improve their quality of life and chance of survival despite their complex condition."
For more information: www.cardiovascular.abbott.com
Related MitraClip Content
MitraClip Reduces Mortality for Heart Failure Patients With Secondary Mitral Regurgitation


 July 31, 2024
July 31, 2024 









