December 21, 2018 — CathWorks announced that its FFRangio System received U.S. Food and Drug Administration (FDA) 510(k) clearance. The FFRangio system demonstrated accuracy versus the invasive fractional flow reserve (FFR) wire in a blinded comparative study, FAST-FFR. The results of the FAST-FFR pivotal study were used to establish substantial equivalence of the FFRangio system.
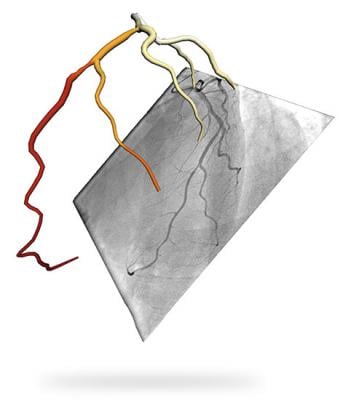
The CathWorks FFRangio System quickly and precisely delivers the objective FFR guidance needed to optimize percutaneous coronary intervention (PCI) therapy decisions. FFRangio is derived from routine X-rays acquired during a diagnostic angiogram procedure, is non-invasive and performed intra-procedurally during coronary angiography, eliminating additional clinical risk, time and cost associated with invasive FFR. The system provides a 3-D reconstruction of the entire coronary tree with FFR values along each vessel.
The FAST-FFR study was carried out at 10 centers worldwide and evaluated more than 380 patients. The study demonstrated the clinical predictive value across a full range of coronary physiology, including complex lesion assessment in bifurcations and calcified lesions. FAST-FFR also demonstrated that the FFRangio system could perform non-invasive, objective, multi-vessel, physiologic measurements to support PCI decision-making
Read more about the trial in the article "FAST-FFR Trial of FFR-angio Technology Meets Primary Endpoint, Exceeded Performance Goals."
Watch the VIDEO: Angiography Image-based FFR May Eliminate Need for Pressure Wires, an interview with William Fearon, M.D.
For more information: www.cath.works

 May 12, 2020
May 12, 2020 









