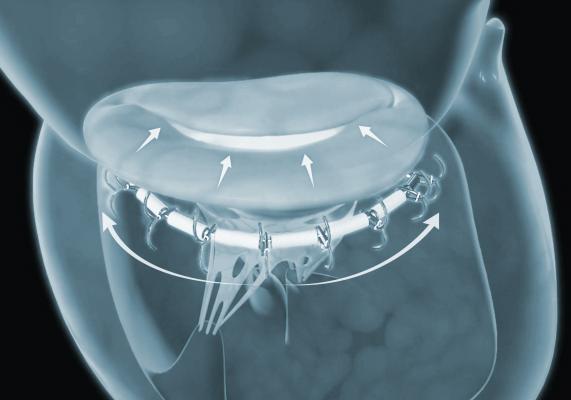
September 24, 2018 — Ancora Heart Inc. announced positive clinical data from the company’s recently expanded U.S. early feasibility study evaluating the safety of the investigational AccuCinch Ventricular Repair System designed for the treatment of heart failure and functional mitral regurgitation (FMR). The data was presented at the 30th Transcatheter Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation, Sept. 21-25 in San Diego.
The transcatheter AccuCinch therapy has the potential to treat heart failure and FMR patients in whom the disease has progressed beyond the ability for medications and pacemakers to manage symptoms, or for whom the risks of open-heart surgery are too high. Unlike current technologies that replicate surgical procedures to replace or repair an otherwise-normal mitral valve, the AccuCinch system is designed to repair the left ventricle of the heart directly, to address the fundamental issue in the progression of systolic heart failure.
An interim analysis from the first cohort of 16 patients with heart failure and FMR was presented in two sessions at TCT 2018 by Dan Burkhoff, M.D., Ph.D., of the Cardiovascular Research Foundation (CRF), and separately by Mark Reisman, M.D., clinical professor of medicine, section head for interventional cardiology, and director of cardiovascular emerging therapies at University of Washington Medical Center.
The initial data from the study, which continues to enroll patients and add trial sites, suggest a favorable safety profile with consistent procedural safety at 30 days, including no major adverse cardiac events and no device-related deaths. Preliminary efficacy data also at 30-day follow-up demonstrated a reduction in left ventricular volume by an average of 29 percent. Ejection fraction (EF), a measure of blood flowing out of the left ventricle, improved on average from 30 percent to 42 percent. In addition, mitral regurgitation grades and regurgitant volumes were both substantially reduced across this cohort.
“We are seeing consistent procedural success and promising safety results with AccuCinch, which is unique in its ventricular approach,” said Burkhoff. “Early efficacy data indicate a mitral regurgitation reduction similar to transcatheter therapies focused on the mitral valve. However, the additional substantial improvement in LVESV and EF in patients treated with the AccuCinch system, which in general is also improving over time, suggests the device may be reshaping the left ventricle, and fundamentally improving heart function. We look forward to continuing to evaluate AccuCinch in this study.”
For more information: www.ancoraheart.com


 January 05, 2026
January 05, 2026 









