May 30, 2018 — Shockwave Medical recently announced the European commercial availability of Intravascular Lithotripsy (IVL) for calcified coronary artery disease (CAD). The company also announced the first patient enrollment in the DISRUPT CAD II post-market study by Prof. Jonathan Hill, M.D., at King’s College in London.
Coronary artery calcium physically impairs stent expansion,[i] and is perhaps the single most important predictor of early stent thrombosis and restenosis after stent procedures.[ii] [iii] [iv] Current calcium modification treatments, which can be difficult to perform, only address the burden of intimal calcium with varying degrees of success. They also result in an increased risk for adverse events since they do not differentiate between the calcific lesion and soft intimal tissue.
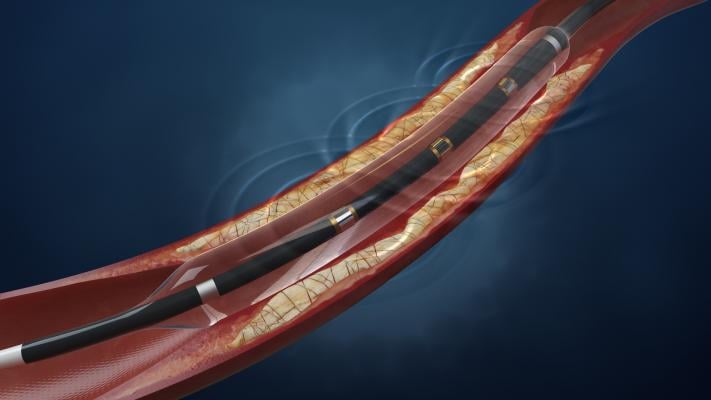
IVL is a novel therapy designed to treat calcified artery blockages with sonic pressure waves historically used to treat patients with kidney stones. The technology minimizes trauma within the artery by delivering pulsatile sonic pressure waves locally to effectively fracture both intimal and medial calcium in the artery wall but pass through surrounding soft vascular tissue in a safe manner. Additionally, IVL requires no specialized training, and it allows physicians to use their own guidewire of choice to seamlessly integrate into the existing workflow.
“For many years, addressing challenging calcium in patients with complex coronary artery disease has been a balancing act, weighing the risk of certain therapies with their clinical benefit,” said Jean Fajadet, M.D., co-principal investigator of the DISRUPT CAD II study and co-director of the Interventional Cardiovascular Group at Clinique Pasteur in Toulouse, France. “Now with the availability of IVL, we finally have an intuitive calcium modification tool that offers the maximum benefit in increasing vessel compliance prior to stent implantation with minimal safety risks.”
The technology previously obtained CE Mark on the strength of the safety and efficacy data in the DISRUPT CAD study. The pre-market, prospective multi-center single arm study was conducted at seven centers in Europe and Australia and enrolled 60 patients with complex calcified CAD. The newly initiated DISRUPT CAD II study is a post-market study that will enroll an additional 120 patients at sites across the globe, including Italy, Germany, Netherlands, Denmark, France, U.K., Spain, Sweden and Belgium.
“Having used Intravascular Lithotripsy in both a clinical trial setting as well as in our everyday clinical practice for complex patients, it’s clear that this is a game-changing technology for the treatment of calcified coronary artery disease,” said Hill. “A more widespread introduction of this technology will significantly augment our ability to modify calcific lesions. It is a highly accessible technology, which is simple to use and can be rapidly deployed in the cath lab.”
The Shockwave Coronary IVL System complements the existing large and small diameter peripheral IVL catheters, which have been available in Europe for the treatment of calcified peripheral disease from the iliac arteries down to the foot since 2015 and early 2018, respectively. The Shockwave Coronary IVL System, similar to the peripheral IVL systems, includes:
- A compact, battery-powered generator;
- A simple and quick handheld connector cable with a single therapy delivery button; and
- An intuitive catheter, which houses an array of lithotripsy emitters enclosed in an integrated balloon. The catheter is delivered to a lesion similar to standard interventional techniques and on the physician’s choice of guidewire.
Shockwave IVL catheters are now commercially available for coronary artery disease in Europe; they are not available in the United States.
For more information: www.shockwavemedical.com
References:


 January 05, 2026
January 05, 2026 









