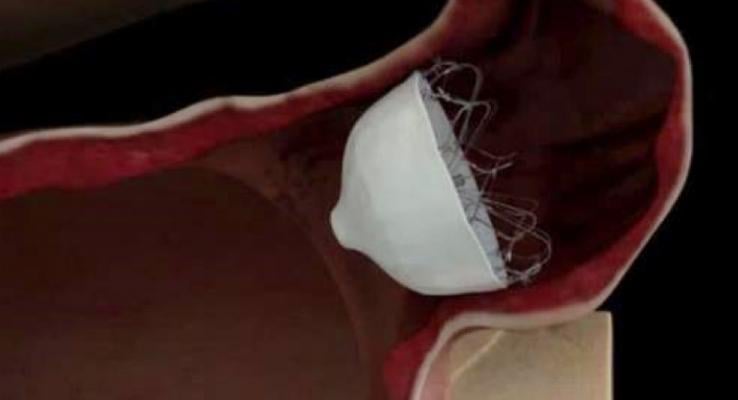
January 18, 2018 – Johnson & Johnson Medical Devices Companies announced that Biosense Webster, Inc., a worldwide leader in the diagnosis and treatment of heart arrhythmias, enrolled the first patient in the WaveCrest Investigational Device Exemption (IDE) Trial. The study will evaluate the safety and effectiveness of the WaveCrest Left Atrial Appendage Occlusion (LAAO) System in closure of the Left Atrial Appendage (LAA) and evaluate reduction of embolic stroke in atrial fibrillation (AFib) patients who cannot tolerate chronic Oral Anticoagulation Therapy (OAC).
The first patient was treated at New York University (NYU) Hospital by Larry Chinitz, M.D., a cardiac electrophysiologist and director of NYU Langone’s Heart Rhythm Center in New York City. The trial will enroll 1,250 patients at approximately 90 hospitals, and follow them for five years.
“The WaveCrest System is designed to enable physicians to close the heart’s LAA, where most stroke-causing blood clots occur,” said Chinitz. “For patients with AFib seeking an alternative to anticoagulants or blood thinners, this may be an important procedural option that could reduce the risk of stroke and save lives.”
According to the American Stroke Association, people with AFib have a five times higher risk of stroke than the general population. The Centers for Disease Control and Prevention (CDC) estimates 87 percent of all strokes are ischemic strokes, in which blood flow to the brain is blocked by the formation of a clot. Stroke is one of the most devastating and debilitating diseases, costing the United States an estimated $34 billion each year.
“Preventing and reducing the risk of stroke in AFib patients is a significant unmet need,” said Shlomi Nachman, company group chairman of Johnson & Johnson Medical Devices Cardiovascular & Specialty Solutions. “We are committed to investing in meaningful innovation and are excited by the prospect of bringing the WaveCrest System to the U.S. market so that more patients can benefit.”
The study is a prospective, multicenter, randomized, active controlled clinical trial of the WaveCrest Left Atrial Appendage Occlusion System compared to an existing FDA-approved LAA Closure Device for the reduction in risk of embolic stroke in subjects with non-valvular atrial fibrillation.
Although the device is under clinical study and not approved for use in the US, the WaveCrest Device is CE Mark approved and available in Europe.
For more information: www.biosensewebster.com


 April 04, 2024
April 04, 2024