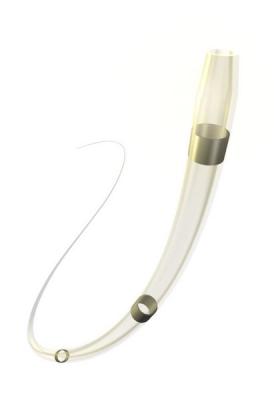
October 12, 2016 — Medtronic plc announced recently that the U.S. Food and Drug Administration (FDA) has cleared the TrailBlazer angled support catheter for use in the peripheral vascular system. Support catheters such as the Trailblazer are often used in endovascular procedures treating complex peripheral artery disease (PAD).
FDA clearance was received on Sept. 23, 2016.
The new catheter is designed to support a guide wire during access to the peripheral arteries, and to enable delivery of solutions and diagnostic agents. The catheter features a braided stainless steel shaft for robust pushability and a 25 and 30 degree angled tapered tip to access and cross complex lesions. To enhance physician visibility, each of the .014-, .018- and .035-inch guidewire-compatible devices is designed with three radiopaque marker bands and a radiopaque shaft. Additionally, both the .014- and .018-inch TrailBlazer angled support catheter can fit coaxially through the .035-inch support catheter for increased reach and pushability.
For more information: www.medtronic.com


 November 14, 2025
November 14, 2025 









