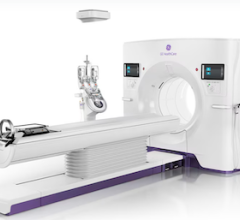
April 19, 2016 — Toshiba America Medical Systems Inc.’s Aquilion Lightning computed tomography (CT) system was U.S. Food and Drug Administration (FDA)-cleared with a more powerful 50 kw generator. The approval means providers can now image a wider range of patients with a low cost of ownership with a reliable, premium-component, entry-level CT system that maximizes their equipment investment.
The Aquilion Lightning is a 16-detector row system designed for routine volumetric scanning. It has a small footprint to allow providers to save on both space and cost, without sacrificing exceptional technology. The system offers the same PureVision CT Detector technology that is used in premium Toshiba CT systems and includes Advanced Iterative Dose Reconstruction (AIDR) 3-D Enhanced to help reduce dose and improve patient safety.
To simplify complex scans for more consistent imaging, the system comes standard with Adaptive Diagnostic solutions, such as Single Energy Metal Artifact Reduction (SEMAR) and SureSubtraction. These technologies — along with what the company calls the industry’s thinnest slices at 0.5 mm, and a 78-cm bore that Toshiba said is the widest in the 16-row detector radiology segment — help optimize workflow and patient comfort.
For more information: www.medical.toshiba.com


 February 02, 2026
February 02, 2026