April 15, 2016 — A new implantable medical device intended to help patients with heart failure by stimulating the vagus nerve did not significantly reduce rates of heart failure-related hospitalization or death from any cause in a study presented at the American College of Cardiology (ACC) 2016 meeting.
The new trial did show benefits of the CardioFit system for some secondary outcomes, including walking endurance and quality-of-life measures. With 707 participants tracked for an average of 16 months, the trial is the first large study with long-term follow-up for any device designed to treat heart failure by modulating the autonomic nervous system, which is the part of the brain that affects heart function. Previous, smaller trials for other such devices have shown mixed results.
“The results are not what we had hoped for, but despite this disappointment, we still feel this approach holds potential. We hope to find new ideas and new refinements in the future, including promising subgroups of patients who may benefit from this treatment,” said Michael Robert Gold, M.D., Ph.D., Michael E. Assey Professor of Medicine at the Medical University of South Carolina in Charleston and the study’s co-principal investigator and lead author.
Heart failure, which affects an estimated 5.1 million people in the United States, is a condition in which the heart progressively weakens and cannot pump enough blood to meet the body’s needs. One of its hallmarks is an imbalanced autonomic nervous system, in which the sympathetic nervous system — the system behind the “fight or flight” response — becomes overactive and the parasympathetic nervous system— behind the “rest and digest” functions — is underactive.
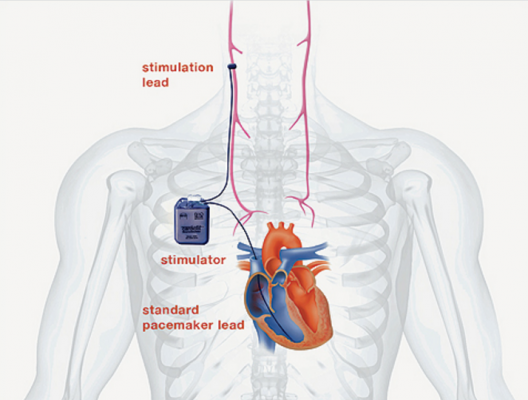
Most available heart failure therapies work by suppressing the overactive sympathetic nervous system and are most beneficial in patients with less severe forms of heart failure. Those with severe and end-stage heart failure often have no options apart from a heart transplant or a left ventricular assist device, a heart pumping machine given to patients who are awaiting a heart transplant. CardioFit was designed to help fill this treatment gap by stimulating the underactive parasympathetic nervous system in patients with severe heart failure. It includes a lead to sense the heart beat, as well as a lead that sends electrical stimulation to a cuff around the vagus nerve, which is the primary conduit between the parasympathetic nervous system and the heart.
The trial enrolled 707 participants with New York Heart Association Class III heart failure at 85 health care centers. Two-thirds were randomly selected for a CardioFit implant, and one-third served as a control group. All participants continued to receive guideline-directed therapy throughout the trial. Because the
CardioFit system creates a sensation that some find uncomfortable, the system was turned off for the first month after implantation and then gradually turned up to allow patients to acclimate to it.
After an average of 16 months of follow-up, the results for the primary endpoints were neutral, showing no significant difference in heart failure related hospitalization or all-cause mortality between those given a CardioFit system and those in the control group. The CardioFit system met pre-specified safety endpoints, indicating no adverse safety concerns as compared to standard care.
Many of the trial’s secondary outcomes showed significant improvement with the CardioFit system. In a test measuring how far a patient can walk in six minutes, those given the CardioFit implant improved distance walked by an average of 28.2 meters over the course of the study, a significant gain as compared to the control group, whose participants were able to walk on average 4.6 meters less at the end of the study than they could at the beginning.
Quality of life, as measured by the Kansas City Cardiomyopathy Questionnaire, which evaluates the degree to which a patient’s heart failure interferes with daily activities, work, recreation and social life, among other factors, was improved 11.2 points in those given the CardioFit system, as compared to 6.9 points in the control group. In addition, 47 percent of patients given CardioFit improved their New York Heart Association heart failure class, significantly greater than 29 percent in the control patients. The study showed no significant difference between the CardioFit and control groups in ejection fraction or the volume of blood pumped by the left ventricle, a measure used to assess the heart’s function.
“Given the encouraging safety outcomes and the tantalizing secondary endpoint data, our study suggests that there may be specific groups of patients who may benefit from this therapy while others may not,” Gold said. “There are a lot more analyses to be done that will help us to develop hypotheses that we may be able to test in future studies. However, it is clear from this study that this device will not benefit all patients with heart failure and a reduced ejection fraction.”
A key limitation of the study was that, because implanting the CardioFit system required a surgical procedure, both patients and physicians were aware of which participants had been randomized to receive CardioFit and which ones were in the control group. It is possible that some of the improvements in secondary outcomes could be attributable to a placebo effect, though it is impossible to assess that effect within this study design.
The trial was funded by BioControl, a company for which Gold serves as a steering committee member.
This study was simultaneously published online in the Journal of the American College of Cardiology at the time of presentation.


 July 31, 2024
July 31, 2024 









