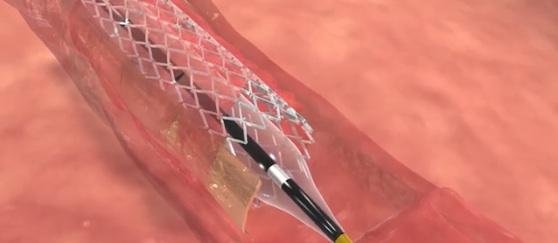
February 8, 2016 — Stentys announced the first distribution agreements for its drug-eluting stent for treating below-the-knee (BTK) arteries in Germany and Belgium, making it the first self-expanding drug-eluting stent commercialized for this indication in Europe.
The Stentys stent obtained CE Marking for the BTK indication at the end of 2015 following the results achieved by the PES BTK-70 study, where it prevented amputation in 99 percent of the 70 patients treated for critical limb ischemia (CLI). The trial treated patients suffering from CLI of class 4 and 5 in the Rutherford scale with a Stentys paclitaxel-eluting stent from January 2012 to May 2013 in five hospitals. The primary endpoint was the 12-month primary patency rate defined as absence of restenosis (≥50 percent) or occlusion within the originally treated lesion based on angiography verified by Core Lab. At 12 months, the primary patency rate was 73 percent, freedom from target-lesion revascularization was 79 percent and freedom from amputation was 99 percent.
For more information: www.stentys.com


 September 12, 2025
September 12, 2025 









