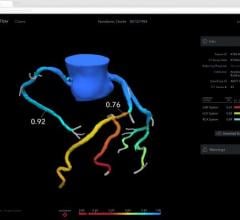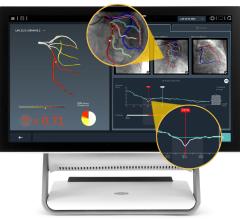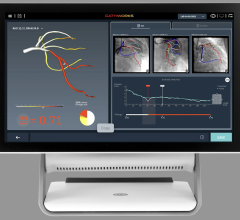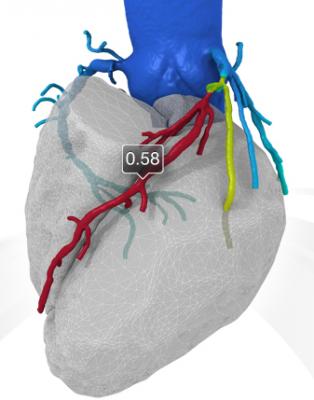
An example of a FFR-CT study showing a 3-D reconstruction of the coronary tree and a color overlay of virtual FFR readings, including a severe blockage in need of revascularization in the left anterior decending artery.
August 21, 2015 — Loyola University Medical Center is the first and only hospital in Illinois to offer a new, noninvasive technology to test for coronary artery disease.
The technology employs noninvasive CT scans to calculate how much blood is flowing through diseased coronary arteries that have narrowed due to a buildup of plaque. The patient does not need to undergo an invasive angiogram that involves threading a catheter to the heart.
The test, developed by HeartFlow Inc., has been approved by the U.S. Food and Drug Administration. It’s called fractional flow reserve-computed tomography (FFR-CT).
“FFRCT provides superior patient care and helps guide treatment strategies with a single, non-invasive study that is low risk and provides accurate information,” said cardiologist Mark Rabbat, M.D., FSCCT, an assistant professor in the departments of Medicine and Radiology of Loyola University Chicago Stritch School of Medicine.
Rabbat said FFR-CT can answer important clinical questions such as whether a patient has coronary artery disease. It can determine whether plaque in a coronary artery is restricting blood flow, thereby helping determine whether a patient would benefit from stents or bypass surgery.
More than 16 million adults in the United States have coronary artery disease. The condition occurs when a buildup of plaque narrows arteries that supply oxygen-rich blood to heart muscle. Reduced blood flow to heart muscles can cause angina (chest pain) and shortness of breath. If an artery becomes completely blocked, a patient can suffer a heart attack.
Blockages that reduce blood flow by a small amount can be treated with medical therapy such as cholesterol-lowering drugs or aspirin. But if there is a major reduction in blood flow, the patient may require a stent or bypass surgery.
Until now, the standard test for measuring FFR involved an invasive angiogram and use of an FFR catheter.
If blood flow is reduced, the blood pressure downstream from the blockage also will be reduced. If this blood pressure is less than 80 percent of the blood pressure in the aorta, a cardiologist may recommend a stent or bypass surgery.
The new FFR-CT technique is noninvasive. CT scans create a digital 3-D model of the arteries leading to the heart. Powerful computer models then simulate the blood flow within those arteries to assess whether blood flow has been restricted by any narrowings. A color-coded map helps physicians determine, vessel by vessel, if sufficient blood is flowing to the heart.
“FFR-CT is a game changer,” Rabbat said. “For the first time, we have a single comprehensive, non-invasive diagnostic test that offers both an anatomic assessment and the functional significance of coronary artery disease. We’re proud to be the first hospital in Illinois to offer this revolutionary technology to our patients.”
For more information: www.loyolamedicine.org


 February 03, 2026
February 03, 2026