May 27, 2015 — Shockwave Medical announced $40 million in funding, co-led by returning investor Sofinnova Partners and new investor Venrock. Also participating were RA Capital, Deerfield, Sectoral Asset Management, Ally Bridge Group and two undisclosed large-cap strategic investors.
Proceeds from the financing will be used for development of the company’s Lithoplasty balloon catheters in peripheral, coronary and aortic valve applications.
The company recently reported six-month follow-up results from the DISRUPT PAD trial, a 35-patient study of patients with calcified vascular stenosis of the superficial femoral artery (SFA) and popliteal artery treated with the Lithoplasty system. Data reported at the 37th annual Charing Cross 2015 Symposium in London demonstrate safe and effective dilatation of calcified stenosis with no acute failures, favorable residual stenosis, no major device-related adverse events and no need for stent placement. Six-month durability was excellent, with no need for re-treatment and patency by duplex ultrasound of 83 percent.
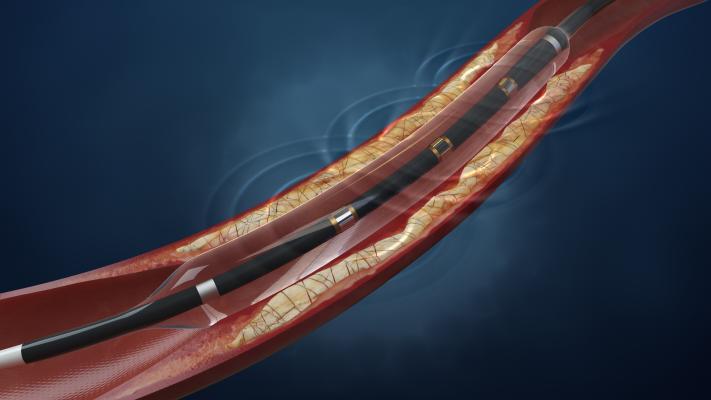
In advanced vascular disease, atherosclerosis becomes calcified deep inside the vessel walls, limiting blood flow. These deposits are difficult to treat because they limit the effectiveness of current endovascular devices, making today’s interventions challenging and prone to both procedural and long-term failure. Lithoplasty is a novel balloon-based technology that utilizes integrated lithotripsy, a pulsatile mechanical energy commonly used to break up kidney stones, to disrupt both superficial and deep calcium and normalize vessel wall compliance prior to low-pressure balloon dilatation. Lithoplasty is designed to be naturally gentle to soft tissue (non-diseased portions of the vessel) while remaining hard on calcium, the tissue that limits vessel expansion and the effectiveness of current technologies.
For more information: www.shockwavemedical.com


 June 13, 2024
June 13, 2024 









