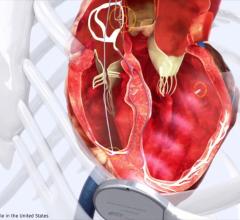
May 19, 2015 — A new epicardial pacing lead has been cleared by the U.S. Food and Drug Administration (FDA) as an option for the implant of a pacemaker, defibrillator, or cardiac resynchronization therapy (CRT) device. The lead is indicated when other types of leads cannot be implanted. Examples include patients who have small veins, congenital heart disease, abnormalities of the tricuspid valve or when other leads are already in place, preventing additional leads in the veins.
The Myopore Sutureless Myocardial Pacing Lead from Greatbatch Medical is a surgically implanted, insulated and sutureless wire with a screw-in tip that is part of a permanently implanted pacemaker, defibrillator or CRT device. The lead is implanted by opening the chest or a making a small incision under the breastbone. It is then placed on the surface of the heart muscle so the screw-in tip end can electrically stimulate (pace) the ventricles.
When placed on the surface of the heart as part of a pacemaker, the Myopore Lead paces the heart to assure that it beats at an appropriate rate. A CRT device uses two leads, one to pace each ventricle, either of which may be a Myopore Lead. A CRT device with two leads is intended to coordinate and improve the ventricles' timing to reduce symptoms of heart failure.
The Myopore Lead should not be used when a patient's heart is too thin or damaged and should not be implanted on the surface of the atria.
For more information: www.greatbatchmedical.com


 July 21, 2025
July 21, 2025