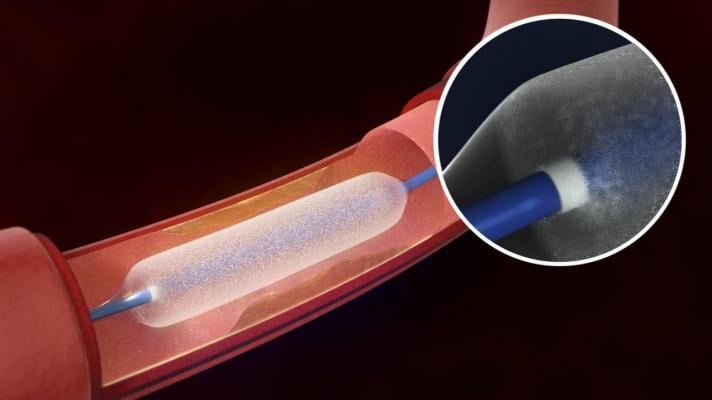
The Bard Lutonix drug-eluting balloon was cleared by the FDA last November.
January 30, 2015 — ECRI Institute has created a report that offers an overview of drug-eluting balloon (DEB) technology, “Health Technology Forecast report, "Drug-eluting Angioplasty Balloons for Preventing Restenosis after Revascularization." The report details some of the key questions regarding DEBs.
Balloon angioplasty to treat peripheral artery disease is associated with high rates of restenosis, or re-narrowing of the arteries. For certain indications, such as long lesions in below-the-knee arteries, the one-year restenosis rate following peripheral angioplasty may be as high as 70 percent. To address this problem, several manufacturers have been developing drug-eluting balloons as an alternative to conventional angioplasty balloons for performing percutaneous transluminal angioplasty, and the first one recently received U.S. Food and Drug Administration (FDA) approval.
Excerpts of the ECRI report are below:
What Do Drug-eluting Balloons Do?
Drug-eluting balloons are designed to deliver their drug dose over several minutes. Most peripheral artery drug-eluting balloons in clinical use or in development are coated with paclitaxel, an antiproliferative drug commonly used in older drug-eluting stents designed for the coronary arteries. The drug inhibits cell proliferation for an extended time and therefore may significantly reduce scar tissue formation in blood vessels. Most current-generation coronary drug-eluting stents are coated with everolimus or zotarolimus. Other antiproliferative agents are also under investigation for use with peripheral drug-eluting balloons.
Widespread Use Depends on Reimbursement
Drug-eluting balloons have been proposed to replace conventional uncoated angioplasty balloons to reduce restenosis rates in treating peripheral artery disease, with or without implantation of a bare-metal stent or drug-eluting stent. ECRI Institute's expert panel commenting on this technology thought that the substantially higher cost of drug-eluting balloons compared with uncoated balloons could limit broader use of the technology unless additional reimbursement becomes available from third-party payers.
Better Outcomes with Drug-eluting Balloons
The expert panel thought that data thus far suggest that drug-eluting balloons may provide better outcomes than uncoated angioplasty balloons in the peripheral arteries. Their use might also reduce the need for repeat intervention to treat re-narrowed arteries and might lessen the anticlotting drug therapy needed after conventional angioplasty for peripheral artery disease. The panel noted that data have not yet shown any definitive effect from this technology on patient outcomes of reduced limb amputation or longer survival.
The Device is Expected to Inflate Hospital Budgets
The panel noted that the anticipated cost of drug-eluting balloons in the United States is up to five times more than conventional angioplasty balloons for peripheral artery disease, but comparable to peripheral drug-eluting stents. Additional reimbursement for drug-eluting balloons (i.e., above the rate reimbursed for uncoated balloon angioplasty) is not available from public and private third-party payers at this time. The panel thought the financial impact could be greater for indications in which permanent stent implantation is less common such as below-the-knee peripheral artery disease. Data suggest that drug-eluting balloons might reduce the need for repeat interventions and lower overall treatment costs compared with conventional peripheral angioplasty, despite the higher initial device costs. If drug-eluting balloons are shown to have comparable long-term efficacy to peripheral drug-eluting stents for reducing restenosis, their use might somewhat reduce costs of treating peripheral artery disease. However, improved outcomes could also reduce hospital revenue if patients need fewer interventions and less care.
For more information: www.ecri.org


 June 13, 2024
June 13, 2024 









