Photo courtesy of Medtronic Inc.
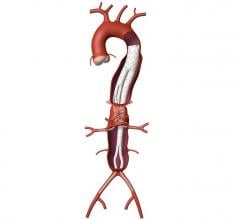
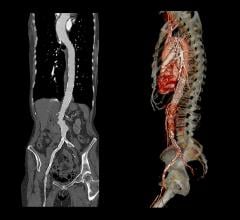
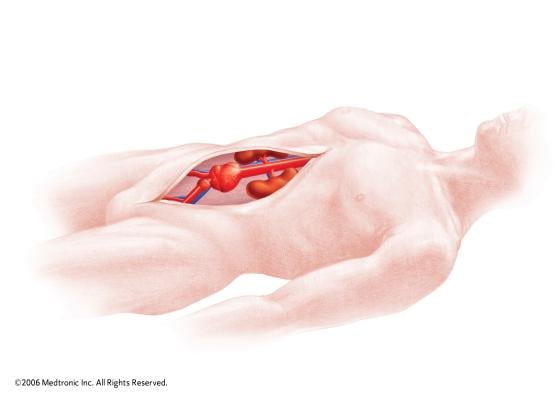
December 10, 2014 — Medtronic Inc. announced the launch in Europe and the United States of the Endurant IIs AAA stent graft, which received CE mark and U.S. Food and Drug Administration approval to be used in the minimally invasive treatment of abdominal aortic aneurysms (AAA).
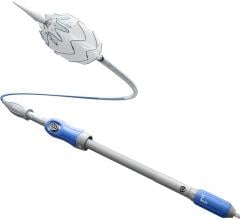
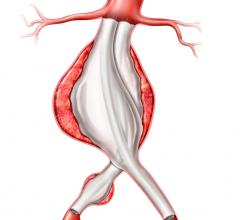
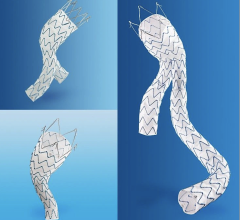
The Endurant IIs stent graft is a new bifurcated component for the system that leverages the design of the Endurant II device and expands the system’s anatomical customization options. The new device is designed as part of a three-piece configuration.
The Endurant IIs stent graft:
- Features equal leg diameters to allow limbs to be used on either side;
- Offers a shorter (50mm) ipsilateral leg for more flexible targeted limb placement;
- Enables in situ sizing with select ipsilateral limbs, allowing a 3–5 stent overlap for adjustment during the implant procedure;
- Provides up to a 20-percent reduction in distal diameter compared to select Endurant II stent grafts; and
- Allows easier pre-case planning by simplifying sizing decisions
The new device complements the existing Endurant II stent graft and uses the same delivery system, which allows for accurate placement and controlled deployment of the device within the aorta.
“Every AAA patient has different anatomical features, which is why it’s so important for a stent graft system to provide for a wide variety of anatomical customization options,” explained William Jordan Jr., M.D., professor of surgery and chief of vascular surgery and endovascular therapy at the University of Alabama Birmingham. “The Endurant stent graft system sets the standard for configuration possibilities, and the addition of the Endurant IIs stent graft expands the possibilities even further.”
For more information: www.medtronic.com

 April 26, 2023
April 26, 2023