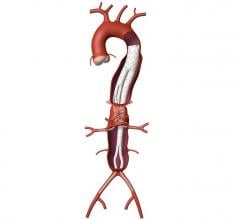
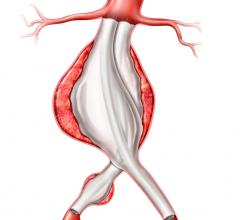
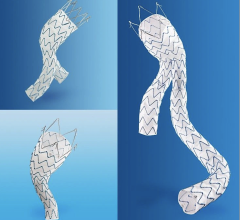
October 22, 2014 — Lombard Medical Inc., a medical device company focused on endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms (AAA), today announced that its lead product, Aorfix, an endovascular stent graft to treat AAA, has received approval from the Japanese Ministry of Health, Labour and Welfare. Commercial sales will follow reimbursement approval. Aorfix will be exclusively distributed by Medico's Hirata Inc. Japan is the world’s second-largest standalone EVAR market.
Aorfix is the first and only endovascular stent graft approved in Japan to treat AAA in patients with aortic neck angulations up to 90 degrees, commonly considered to be challenging cases. Aorfix is currently approved to treat patients with neck angles up to 90 degrees in the U.S. and Europe.
The EVAR market in Japan is estimated at $140 million, or 10 percent of the global market in 2013, and has been growing at an average rate of 18 percent over the last five years. In Japan, there are approximately 400 physicians at 200 clinics performing EVAR and it is estimated that approximately 55 percent of Japanese AAA patients are treated using this method.
For more information: www.lombardmedical.com

 April 26, 2023
April 26, 2023