LoneStar Heart Inc.Algisyl-LVR Hydrogel Implant Heart Failure Treatment CE Mark
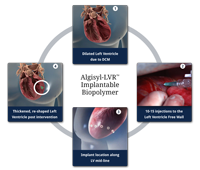
October 10, 2014 — LoneStar Heart Inc. announced that it received the CE mark (Conformite Europeene) for its Algisyl-LVR hydrogel implant, the company's lead product for the treatment of advanced heart failure (HF). Classified in Europe and in the U.S. as a medical device, Algisyl-LVR is intended to reverse HF progression in people who have an enlarged left ventricle. Surgically injected directly into the heart muscle, the hydrogel acts immediately as an internal scaffold that does not undergo long-term degradation and increases cardiac output.
The CE mark indicates a product's compliance with European Union legislation and that it can be sold throughout the European Economic Area. Six-month patient outcome studies of Algisyl-LVR will be presented at the annual meeting of the American Heart Assn. (AHA), Nov. 15-19, 2014 in Chicago.
"CE marking is a major milestone our clinicians have worked hard to achieve," said Frank Ahmann, LoneStar Heart's president and chief operating officer. "Thanks to them, patients may soon have a revolutionary treatment to reduce the symptoms of moderate to severe heart failure and provide improvement in their clinical status and quality of life. To date, our safety and efficacy results are consistently trending in the right direction, and we are looking forward to presenting the results of our AUGMENT-HF randomized clinical trial of Algisyl-LVR at the upcoming AHA meeting in November."
The AUGMENT-HF randomized clinical trial is being conducted at 14 centers in Italy, Germany, Romania, Australia and The Netherlands to determine if the product is superior to standard medical therapy in the management of patients with a dilated and weakened left ventricle and significantly deteriorated cardiac function.
As reported last November 2013 at the American Heart Assn. annual meeting in Dallas, interim results of the study showed left ventricle augmentation of the failing heart after implanta¬tion with Algisyl-LVR can be performed safely in patients with advanced heart failure and provides functional improvement in their health status. Extensive preclinical studies have shown Algisyl-LVR decreases cardiac wall tension, while it improves heart muscle contractility and oxygen uptake, leading to a decrease in ventricle stress and to a marked cardiomechanic improvement.
For more information: www.lonestarheartinc.com


 February 03, 2026
February 03, 2026 









