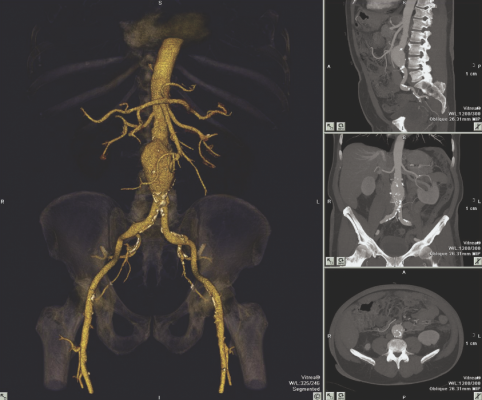
August 20, 2014 — Vital Images Inc. received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Image Denoising software, a post-processing filter designed to be used in conjunction with original image data used with its Vitrea software.
Vital's denoising filter, Structure Preserving Diffusion (SPD), focuses on increasing image quality by improving the appearance of noisy images for computed tomography (CT) and X-ray angiography. It addresses the noise challenges presented by today's CT protocols. SPD can be used with any scanner generation in conjunction with iterative reconstruction (IR) techniques to enhance diagnostic interpretation. Denoising filters are applied in all available viewports; e.g., MPR (multiplanar reformat), CPR (curved planar reformat), 3-D and other volumetric views. Denoised series can then be stored with the original source images in picture archiving and communications systems (PACS). Built to work with existing hardware, users have the ability to create and save their own denoising filters.
"Image Denoising helps customers see a clear view of anatomy from noisy scans without changing the integrity of the image," said Jim Litterer, general manager at Vital. "In terms of efficiency and productivity, denoising is a game-changer. Technologists no longer have to spend hours sorting through the noise to get clear images."
Image Denoising is available in the United States in Vitrea version 6.6.3 and in previous Vitrea versions in other countries globally.
For more information: www.vitalimages.com

 May 12, 2020
May 12, 2020 









