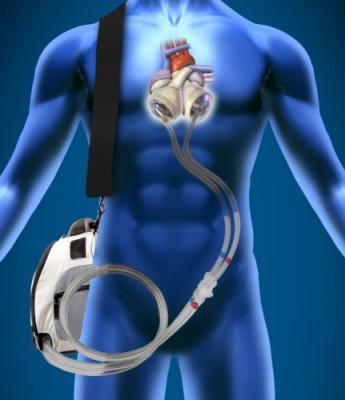
SynCardia Systems Inc. received FDA approval to manufacture the SynCardia Total Artificial Heart with SynHall valves. The OK gives the company control over the two critical components for heart manufacturing: The valves and the unique formula for segmented polyurethane solution (SPUS), which is manufactured only by SynCardia.
August 11, 2014 — SynCardia Systems Inc. received approval in July from the U.S. Federal Drug Administration (FDA) for the SynCardia temporary Total Artificial Heart with SynHall valves, giving the company control over the last key component to manufacture the Total Artificial Heart.
FDA approval follows SynCardia’s receipt of the CE mark for European use of the SynHall valves only in the SynCardia Total Artificial Heart in April 2014. Health Canada approval was received for the same purpose in July.
The SynHall valves — only for use in the SynCardia Heart — are of the same design, materials and nearly identical manufacturing processes as the tilting-disk valves that have always been used in the Total Artificial Heart.
Made of titanium and pyrolytic carbon, these robust valves have never failed in more than 1,300 implants of the SynCardia Total Artificial Heart, accounting for more than 5,000 valves and more than 30 years of use.
SynCardia also controls manufacture of segmented polyurethane solution (SPUS), which is used in the SynCardia Total Artificial Heart’s housings, diaphragms and connectors. SynCardia is the only source of SPUS in the world.
For more information: www.syncardia.com


 May 30, 2025
May 30, 2025 









