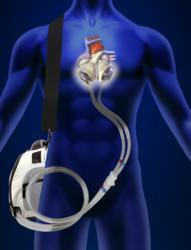
July 18, 2014 — The Freedom portable driver has received U.S. Food and Drug Administration (FDA) approval for use with the SynCardia temporary total artificial heart as a bridge to transplantation in cardiac transplant candidates who are clinically stable.
“With the FDA approval letter for the Freedom portable driver, SynCardia certified centers may now use the Freedom portable driver with all of their clinically stable patients,” said Michael Garippa, SynCardia CEO and president. “These patients can be discharged and live in their homes and communities while they wait for their matching donor hearts. We anticipate that this will save patients, hospitals and insurance companies thousands of dollars through eliminating most in-hospital costs for this portion of patient care.”
The FDA premarket approval study (PMA) of the Freedom driver excluded patients needing IV therapy or outpatient dialysis, Garippa explained. With the FDA approval of the Freedom PMA study, stable patients who need IV therapy and/or outpatient dialysis will now be able to be discharged from the hospital with the Freedom driver and take care of those conditions as needed as a walk-in patient.
The PMA involved 22 SynCardia certified centers in the United States that provided the Freedom driver to 96 SynCardia total artificial heart patients. An additional 10 patients were given the Freedom under compassionate-use exemptions at nine other centers.
Those 106 patients received 58 total years of support starting when they were first switched to the Freedom portable driver in the U.S. study. The SynCardia total artificial heart with the Freedom drive system allowed 75 percent of those patients to be discharged from the hospital, while 86 percent of the 106 patients either were bridged to heart transplants or were alive and supported by the system as of June 30, 2014.
“Most Freedom patients are free to exercise, eat out at restaurants, sleep in their own beds and socialize, all of which help contribute to better outcomes for these patients’ human heart transplants,” Garippa said.
The 13.5-pound Freedom driver can be carried in a backpack, shoulder bag, walker or a rolling caddy. It powers the total artificial heart with precisely calibrated pulses of air and a small amount of vacuum so that the diaphragm is in the proper position to accept the next filling of blood into each artificial ventricle.
Like a heart transplant, the SynCardia total artificial heart is designed to eliminate the source of end-stage biventricular failure by replacing both failing ventricles and the four heart valves of a patient.
The Freedom runs on lithium-ion batteries that are re-charged using a standard electrical outlet or a car charger that can be used in autos and many boats.
The Freedom was approved in Europe in March 2010 and in Canada in May 2011.
For more information: www.syncardia.com


 May 30, 2025
May 30, 2025 









