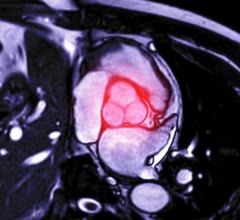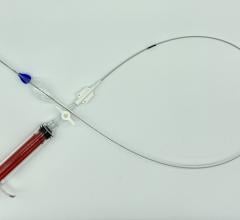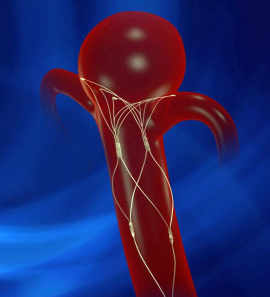
June 20, 2014 — Pulsar Vascular announced it received U.S. Food and Drug Administration (FDA) investigative device exemption (IDE) approval for the PulseRider, a minimally invasive aneurysm neck reconstruction device. The IDE allows Pulsar Vascular to begin a multicenter clinical trial in support of a humanitarian device exemption (HDE) to evaluate the PulseRider for U.S. approval for wide neck aneurysms at or near a bifurcation of the basilar tip or carotid terminus. There are currently no devices approved by the FDA for this indication.
Adnan Siddiqui, M.D., University of Buffalo, said, "The treatment of bifurcation aneurysms is truly an unmet need in endovascular therapies. I am excited by the positive response to the PulseRider in Europe, and as part of the U.S. clinical trial, I look forward to working with the company and the FDA to bring this novel, innovative technology into my everyday practice."
Rob Abrams, the CEO of Pulsar Vascular and co-creator of the PulseRider design, said, "The FDA's approval of the PulseRider IDE allows us to initiate this important study for our flagship product and validates our scientific platform technology. This approval represents another significant milestone for Pulsar Vascular and a step forward on the path to providing a new treatment option to both patients and physicians."
The clinical trial will be conducted at multiple clinical sites in the United States and was slated to begin in the third quarter of 2014.
The PulseRider, which is designed to be fully retrievable and repositionable, received CE mark in late 2013 and has been in use in Europe since early 2014. The device addresses an unmet clinical need to treat complex necked bifurcation aneurysms, and the European physicians have welcomed it into their practices. The company plans to expand the European market while the U.S. clinical trial is underway.
For more information: www.pulsarvascular.com


 September 18, 2025
September 18, 2025