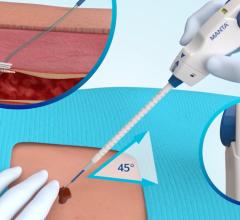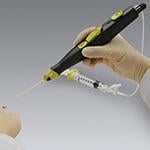
June 13, 2014 — Cardinal Health announced the 2 millionth shipment of the Mynx Vascular Closure Device (VCD). The Mynx product family of vascular closure devices combines the reliability of an easy-to-use deployment system with the security of mechanical closure and the safety of an extravascular sealant that enables physicians to seal an arteriotomy securely without leaving anything permanently behind in the artery.
“There are several vascular closure devices on the market, but most of them leave material behind, either inside the femoral artery, like a plug, or material in the arterial wall, like a suture or clip, which limits their use in certain situations,” said Najam Wasty, director of invasive cardiovascular laboratory and director of Interventional Cardiology Fellowship Training Program at a major teaching institution. “The major advantage of the Mynx VCD is that it is extravascular. In other words, it does not leave any materials permanently inside the artery or in the wall of the artery. Many physicians find this feature attractive since it allows safe use of the Mynx VCD in most situations.”
The first Mynx Vascular Closure Device received U.S. Food and Drug Administration (FDA) approval in May 2007. The Mynx product family includes the new Mynx Ace Vascular Closure Device, launched in 2013 in the United States, and the MynxGrip Vascular Closure Device, launched in 2012. Mynx devices use proprietary Grip Technology, which employs an extravascular sealant that actively adheres to the artery for safe and secure mechanical closure and dissolves within 30 days, leaving nothing permanently behind in the healed artery.
Cardinal Health completed its acquisition of AccessClosure in May.
The Mynx product family is clinically proven and has been featured in several peer-reviewed articles.
For more information: www.accessclosure.com


 October 07, 2025
October 07, 2025