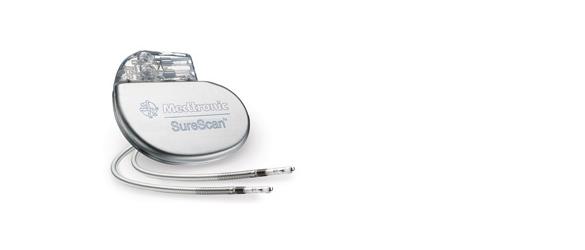
March 3, 2014 — The Medtronic SureScan pacing systems are now approved for MRI scans positioned on any region of the body. The U.S. Food and Drug Administration (FDA) previously approved the SureScan for use with magnetic resonance imaging (MRI). Patients implanted with the Advisa DR MRI or Revo MRI SureScan pacing systems now can have MRI scans without positioning restrictions, including the chest area, which was previously restricted.
Until the availability of Medtronic’s SureScan pacemakers, U.S. patients had been contraindicated from receiving MRI scans due to potential interactions between the MRI and device function. According to published literature, up to 75 percent of patients worldwide with implanted cardiac devices are estimated to need an MRI scan during the lifetime of their devices.
The approval was made following the FDA review of computer modeling and clinical data confirming that MRI chest-positioned scans are safe for patients. A MR-Conditional pacemaker, the Medtronic Revo MRI was FDA approved in February 2011, and the second-generation Advisa MRI was approved by the FDA in January 2013.
For more information: www.medtronic.com


 January 29, 2026
January 29, 2026 









