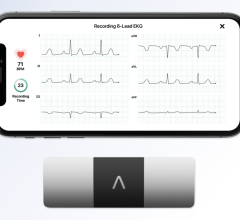
December 11, 2012 — AliveCor Inc. announced at the start of the 4th annual mHealth Summit in Washington, D.C., that it has received CE mark and U.S. Food and Drug Administration (FDA) 510(k) clearance on its mobile Heart Monitor. This clinical-quality, low-cost mobile electrocardiogram (ECG) heart monitor, compatible with the iPhone 4 and 4S, enables doctors to evaluate patient heart health easily, quickly and remotely.
Clinical studies of the device indicate that a high-quality single-channel ECG can be rapidly and simply recorded using an iPhone with the AliveCor application and device, to accurately screen for cardiac arrhythmias, including atrial fibrillation. Atrial fibrillation is the most commonly occurring arrhythmia and carries a five-fold increased risk of stroke.
Additionally, AliveCor’s founder, David Albert, and co-founders Bruce Satchwell and Kim Barnett were granted U.S. Patent No. 8,301,232 for the device and technology. The three colleagues began working on the heart monitoring device in 2008. “We believe that mobile ECGs and other breakthroughs in mobile health can significantly change the way medicine is delivered,” Albert said.
AliveCor’s Heart Monitor is initially intended for use by licensed medical professionals to record, display, store, transfer and evaluate single-channel ECG rhythms.
The rhythm strips can be of any duration, and are stored on the iPhone and securely in the cloud for later analysis, sharing and printing through AliveCor’s secure website. The ECG data is sent wirelessly from the Heart Monitor via AliveCor’s low-power, proprietary communication protocol, and requires no pairing between the iPhone and the device.
The device incorporates electrodes into a case that snaps onto the back of an iPhone 4 or 4S. The Heart Monitor is used by launching the corresponding AliveECG app on the iPhone, holding the device in a relaxed state, and pressing fingers from each hand to each of the two appropriate electrodes on the device. The device can also be used to obtain an ECG by placing it on the chest.
“Our goal is to make healthcare cheaper, easier and more readily available without losing quality of care,” said AliveCor president and CEO Judy Wade. “Our aspirations are significant; we’re out to make a real difference.”
AliveCor’s Heart Monitor is available for pre-sale to medical professionals in the United States through the company website at a cost of $199. AliveCor plans to begin selling the Heart Monitor in Europe in early 2013.
For more information: www.alivecor.com


 September 16, 2025
September 16, 2025