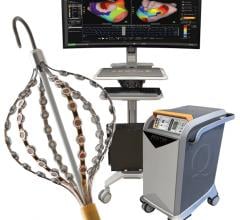
October 19, 2012 — Boston Scientific Corp. entered into a definitive agreement to acquire privately-held Rhythmia Medical Inc., a developer of next-generation mapping and navigation solutions for use in cardiac catheter ablations and other electrophysiology (EP) procedures, including atrial fibrillation and atrial flutter. Rhythmia Medical is based in Burlington, Mass. The transaction was expected to close Oct. 12.
"The acquisition of Rhythmia Medical is a decisive step forward for Boston Scientific in the electrophysiology ablation business, including the high-growth segment of complex ablation," said Hank Kucheman, CEO of Boston Scientific. "Electrophysiology is a $2.5 billion market and growing at a double-digit pace, representing a key growth opportunity for us. Rhythmia Medical has a strong and impressive team, and its technology is expected to add innovation and breadth to Boston Scientific's suite of solutions in this strategically important space."
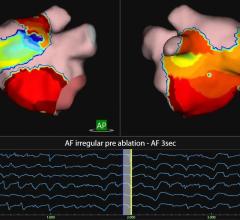
Atrial fibrillation is a disorder that disrupts the ability of the heart to beat regularly and pump blood efficiently. Approximately 15 million people worldwide are affected. Catheter ablation enabled by 3-D mapping and navigation is commonly used to treat many heart rhythm disorders, including atrial flutter and atrial fibrillation.
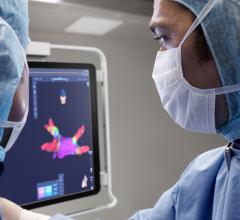
"Rhythmia Medical's revolutionary mapping technology is expected to significantly enhance physician treatment options and ultimately facilitate and improve what today are long and complicated procedures," said Doron Harlev, co-founder and co-CEO of Rhythmia Medical. "Our system is expected to become a very promising tool for physicians to treat patients with complex cardiac arrhythmias. We are excited to combine our mapping system with Boston Scientific's strong catheter platform and commercialization capabilities."
Once the mapping system is cleared by the U.S. Food and Drug Administration (FDA) and receives CE mark approval in Europe, Boston Scientific expects to begin a limited market launch of the system in 2013 and a full market launch in 2014.
The agreement calls for an upfront payment of $90 million payable upon transaction closing, and up to an additional $175 million in contingent payments based on regulatory, commercial and sales-based milestones through 2017. Boston Scientific currently expects the net impact of this transaction on adjusted earnings per share to be immaterial for years 2013 and 2014 and break even to accretive thereafter, and more dilutive on a GAAP basis as a result of acquisition-related net charges and amortization, which will be determined during the fourth quarter.
For more information: www.bostonscientific.com


 September 05, 2025
September 05, 2025