
October 8, 2012 — The U.S. Food and Drug Administration (FDA) has granted Boston Scientific Corp. regulatory approval for its S-ICD System, the world's first commercially available subcutaneous implantable cardioverter defibrillator (S-ICD) for the treatment of patients at risk for sudden cardiac arrest (SCA). The S-ICD System sits entirely just below the skin without the need for implantable lead to be placed inside the heart. This leaves the heart and blood vessels untouched, offering patients an alternative to transvenous ICDs, which require leads to be placed in the heart itself.
"The S-ICD System establishes the first new category of cardiac rhythm management devices since the introduction of cardiac resynchronization therapy," said Raul Weiss, M.D., associate professor of clinical and cardiovascular medicine at The Ohio State University. "Doctors now have a breakthrough treatment option that provides protection from sudden cardiac arrest without touching the heart."
Approval of the S-ICD System was based on data from a 330-patient, prospective, nonrandomized, multicenter clinical study, which evaluated the safety and effectiveness of the system in patients at risk of SCA. The system met the primary endpoints of the study, and results were presented earlier this year at the Heart Rhythm Society (HRS) 33rd Annual Scientific Sessions. The study results support that the S-ICD System is an important new treatment option for a wide range of primary and secondary prevention patients.
"With the addition of the S-ICD System, we believe Boston Scientific has a compelling and highly differentiated portfolio that will help fuel our growth strategy," said Hank Kucheman, chief executive officer, Boston Scientific. "We are the only company to offer an FDA-approved subcutaneous implantable defibrillator and expect this to be the case for several years.
Sudden cardiac arrest is an abrupt loss of heart function. Most episodes are caused by the rapid and/or chaotic activity of the heart known as ventricular tachycardia or ventricular fibrillation. Recent estimates show that approximately 850,000 people in the United States are at risk of SCA and indicated for an ICD device, but remain unprotected. Part of the reason is the more complicated procedure required to implant transvensous leads into the heart. These leads also wear out and require replacement, possibly several times over a patient's lifetime.
"Each year, thousands of patients indicated for an ICD are not referred to a specialist and remain untreated," said William T. Abraham, M.D., FACC, director, division of cardiovascular medicine at The Ohio State University Heart Center. "The S-ICD System is an important new treatment option that has the potential to improve patient acceptance of ICD therapy."
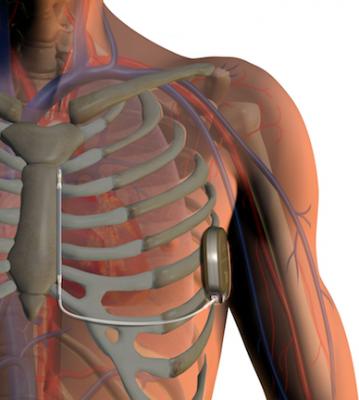
The S-ICD System is designed to provide the same protection from sudden cardiac arrest as transvenous ICDs. The system has two main components: (1) the pulse generator, which powers the system, monitors heart activity and delivers a shock if needed, and (2) the electrode, which enables the device to sense the cardiac rhythm and deliver shocks when necessary. Both components are implanted just under the skin — the generator at the side of the chest, and the electrode beside the breastbone. Unlike transvenous ICDs, the heart and blood vessels remain untouched. Implantation with the S-ICD System is straightforward using anatomical landmarks, without the need for fluoroscopy, eliminating patient X-ray exposure. Fluoroscopy is required for implanting the leads attached to transvenous ICD systems.
Boston Scientific expects to begin a phased launch of the S-ICD System that will expand over time as medical professionals are trained on the safe and effective use of the system. The company acquired the S-ICD System earlier this year when it completed the acquisition of Cameron Health Inc. The S-ICD System received CE mark in 2009 and is commercially available in many countries in Europe as well as in New Zealand. To date, more than 1,400 devices have been implanted in patients around the world.
The S-ICD System is intended to provide defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia, incessant ventricular tachycardia, or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing.
For more information: www.bostonscientific.com


 January 29, 2026
January 29, 2026 









