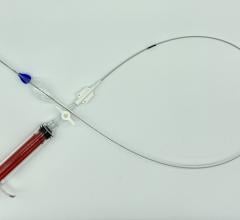
June 8, 2012 — Medtronic Inc. announced the U.S. launch of the Endurant II AAA Stent Graft System, which recently received approval from the U.S. Food and Drug Administration (FDA) for the minimally invasive treatment of abdominal aortic aneurysms through endovascular repair (EVAR), an alternative to major surgery.
The Endurant II AAA System will be showcased at the Society for Vascular Surgery’s Vascular Annual Meeting June 7–9 at National Harbor, Md., near Washington, D.C. Clinical performance updates on Medtronic’s entire portfolio of aortic stent grafts will also be presented at the meeting.
An abdominal aortic aneurysm (AAA) is a condition in which the aorta bulges or weakens, often with no apparent symptoms. An untreated AAA can rupture unexpectedly, which often results in sudden death, making this condition known as a “silent killer” and the third leading cause of sudden death in men over age 60.
The Endurant II AAA Stent Graft builds on the proven performance of the market-leading Endurant AAA Stent Graft, while adding three distinct features that enhance the device’s ease of use and options for clinical success:
- A lower-profile delivery system with an extended hydrophilic coating improves access. The 28-mm-diameter stent graft (the most commonly used size) now fits inside an 18-French-OD (outer diameter) catheter (down from 20 French in the original device).
- Two new contralateral limb lengths (156 mm and 199 mm) provide more options in sizing and can reduce the number of pieces required for an EVAR case.
- Improved radiopacity of the contralateral gate on the distal end of the device aids with limb insertion, placement and deployment.
“The Endurant II Stent Graft is a very deliverable device that enhances the ability to treat more patients than previous devices and to traverse challenging anatomies, especially iliac arteries with tight access,” said William Jordan, M.D., professor and chief of vascular surgery and endovascular therapy at the University of Alabama, and one of the principal investigators for the U.S. clinical study of the predicate device. “Considering the exceptional clinical performance of the original system, the Endurant II Stent Graft is even easier to use in both straightforward and challenging anatomies.”
The original Endurant AAA Stent Graft received FDA approval in December 2010. The next-generation Endurant II AAA Stent Graft received CE mark in December 2011 and has replaced the preceding version in western Europe, while maintaining the preeminent market position that had been held by the Endurant AAA Stent Graft since 2008. Overall, 70,000 patients worldwide have been treated with the Endurant product line.
FDA approval of the Endurant II AAA Stent Graft is based on the results of the U.S. clinical study of the predicate device, which continues to demonstrate strong clinical performance and efficacy in long-term follow-up. The outcomes at twoyears showed no Type-I endoleaks, migration, conversion to surgery, or aneurysm-related mortality. Furthermore, aneurysm sac diameter decreased or remained stable in 98.3 percent of patients at two years.
For more information: www.medtronic.com


 September 18, 2025
September 18, 2025