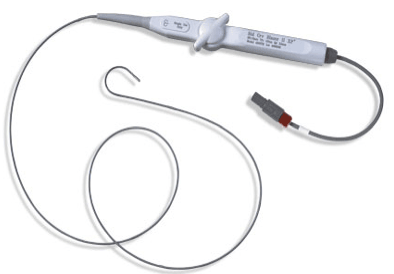
March 16, 2012 — Boston Scientific Corp. announced Health Canada approval of the Blazer open-irrigated catheter, the company's latest radiofrequency ablation (RFA) catheter designed to treat a variety of arrhythmias such as atrial fibrillation (AF), atrial flutter, ventricular tachycardia and other supraventricular tachycardias. The product will be launched immediately in Canada.
The Blazer open-irrigated catheter integrates Total Tip Cooling design with the high-performance Blazer catheter platform. The Total Tip Cooling design consistently cools the entire tip electrode during radiofrequency (RF) energy delivery, resulting in reduced coagulum at the proximal edges of the tip. This is achieved through both internal cooling and external washing of the tip electrode.
"The excellent cooling profile, coupled with the high performance Blazer platform, makes this catheter an important additional option for performing simple and complex ablations," said Felix Ayala-Parades, M.D., chief electrophysiologist at Sherbrooke University Hospital Centre in Quebec, who performed the first procedure in Canada using the Blazer open-irrigated catheter with Santiago Rivera, M.D. "The tip temperatures were lower than conventional open-irrigated catheters during RF delivery. The catheter handling also allowed for a smooth drag lesion to be created without any gaps in the lesion set and with good contact to ensure adequate lesion creation."
AF is a rapid irregular rhythm affecting the upper chambers of the heart. Patients are most often treated with anti-arrhythmic drugs, which can be associated with adverse side effects. Cardiac ablation with an radiofrequency ablation (RFA) catheter is being used increasingly for patients who cannot tolerate these medications.
"The Health Canada approval and launch of the Blazer open-irrigated catheter in Canada are important milestones in our continued focus on advancing cardiac ablation technology," said Hank Kucheman, CEO of Boston Scientific. "We are committed to expanding our Canadian electrophysiology business and improving outcomes for patients undergoing cardiac ablation procedures."
In the United States, the blazer open-irrigated catheter is an investigational device, limited by applicable law to investigational use only and not available for sale. The product is approved for use in CE mark countries.
For more information: www.bostonscientific.com


 February 06, 2026
February 06, 2026 









