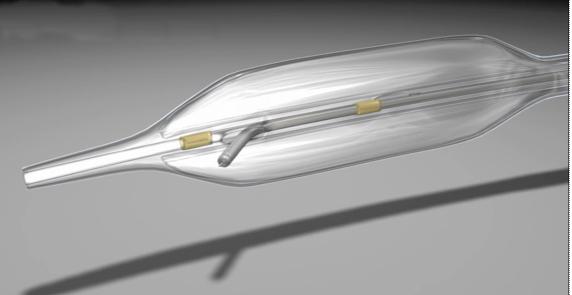
January 20, 2012 - BridgePoint Medical Inc. this week signed an agreement with Covidien to exclusively license the rights to its peripheral vascular products used to treat chronic total occlusions (CTO). The agreement includes the BigBoss and Mantaray catheters and the Mantaray guidewire. The terms of the agreement were not disclosed.
“This is a fantastic achievement for BridgePoint Medical,” said Denis Harrington, CEO and president, BridgePoint Medical. “We are pleased with this transaction and expect that this agreement will be a positive development for all BridgePoint shareholders.”
The bulk of BridgePoint Medical's assets including the coronary chronic total occlusion (CTO) products, intellectual property, its employees and all other non-vascular applications will remain with the company.
BridgePoint Medical, Inc. is a privately held company established in 2006 to design, develop and commercialize new technologies and techniques to treat challenging coronary artery disease.
The Mantaray catheter was cleared by the U.S, Food and Drug Administration in August 2011. The BigBoss cathter was cleared in January 2012.
For more information: www.bridgepointmedical.com


 October 28, 2025
October 28, 2025 









