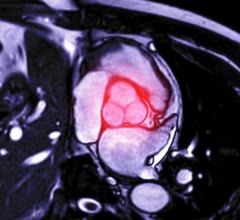
November 23, 2011 – The U.S. Food and Drug Administration (FDA) allowed marketing of the first system that can repair a failed or problematic aortic endograft, a fabric tube used to repair a dangerously large aortic aneurysm, a bulge in the large blood vessel that carries blood away from the heart.
FDA's action will provide surgeons with a minimally invasive option for repair of aortic endografts (endovascular grafts) that are not properly positioned. Aortic aneurysms can, over time, become weak and result in a life-threatening rupture. The endograft is placed inside the aorta to seal off the aneurysm and direct blood away from it.
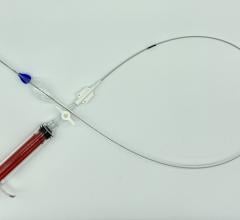
The Aptus EndoStapling System is a cassette of nickel-cobalt corkscrew-shaped staples that is loaded into a long, thin, tube-like delivery catheter. The catheter is inserted into an artery in the leg and directed through the arteries to the failed endograft. Using a controller on the handle of the catheter, the surgeon applies one staple at a time around the top edge of the endograft to anchor the device and repair the endograft-artery seal.
"Leakage between the top end of the endograft and the aorta wall is a known complication of endograft implants that can be successfully treated," said Christy Foreman, director of the Office of Device Evaluation in the FDA's Center for Devices and Radiological Health. "The Aptus EndoStapling System provides a less invasive option than open surgery to access and repair these leaks."
The FDA reviewed data for the Aptus EndoStapling System through the de novo reclassification process, a regulatory pathway for low- to moderate-risk medical devices that are novel and not comparable to an already legally marketed device.
FDA granted the de novo petition for the EndoStapling System based on review of data from 154 patients who were implanted with 810 EndoStaples. Patients were monitored with routine follow-up computed tomography (CT) scans. After a year, none of the EndoStaples had fractured and no patients experienced endograft movement (migration); one subject needed an additional intervention to address an endoleak.
The Aptus EndoStapling System is for use in patients whose endovascular grafts have moved, exhibit endoleaks, or are at risk for these complications and additional intervention is needed to reattach the graft and seal off the aneurysm.
For more information: www.aptusendosystems.com/


 September 18, 2025
September 18, 2025