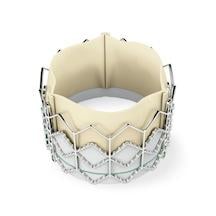
November 2, 2011 - The U.S. Food and Drug Administration (FDA) today approved the first transcatheter aortic heart valve in the United States - the Sapien THV. Experts say the approval will open a new chapter in how patients with severe aortic valve stenosis will be treated, without surgery.
Senile aortic valve stenosis is a progressive, age-related disease caused by calcium deposits on the aortic valve that cause the valve to narrow. As the heart works harder to pump enough blood through the smaller valve opening, the heart eventually weakens, which can lead to problems such as fainting, chest pain, heart failure, irregular heart rhythms (arrhythmias) or cardiac arrest. Once symptoms of senile aortic stenosis occur, more than half of patients die within two years. To restore normal blood flow, patients with severe aortic valve stenosis need open-heart surgery to replace the diseased valve. However, the procedure is too risky for some patients.
“Surgery to replace the aortic valve is an effective treatment for severe senile aortic valve stenosis. The Sapien Transcatheter Heart Valve (THV) is an example of an innovative new device that will provide some people with this condition who can’t undergo open heart surgery with the option of valve replacement,” said Jeffrey Shuren, M.D., director of the FDA’s Center for Devices and Radiological Health. “The agency remains committed to working with companies who are developing breakthrough treatments that will have a significant impact on patient care in the U.S.”
The Sapien is made of cow tissue and polyester supported with a stainless steel mesh frame. To replace the diseased valve, the Sapien is compressed into the end of a long, thin, tube-like device called a delivery catheter. The delivery catheter, which is slightly wider than a pencil, and the Sapien are inserted into the femoral artery through a small cut in the leg and threaded to the site of the diseased valve. The heart valve is then released from the delivery catheter and expanded with a balloon and is immediately functional.
Transcatheter aortic valve replacement (TAVR) with the Edwards Sapien valve enables multi-disciplinary heart teams to replace a patient's diseased aortic valve without traditional open-heart surgery and while the heart continues to beat - avoiding the need for cardiopulmonary bypass.
"This day marks an important milestone for inoperable American patients who have long been awaiting a therapeutic option for the often debilitating symptoms associated with severe aortic stenosis," said Michael A. Mussallem, Edwards' chairman and CEO. "We are extremely proud of the dedication of the heart teams and the patients involved in the clinical trial for this therapy, who have paved the way for this therapy to help even more people around the world."
In performing the TAVR procedure, the valve is crimped onto the catheter-based transfemoral delivery system, which is inserted into the body through a small cut in the leg. Once delivered to the site of the patient's diseased valve, the Edwards Sapien valve is expanded with a balloon and immediately functions in place of the patient's native valve.
TheSapien valve is indicated for transfemoral delivery in patients with severe symptomatic native aortic valve stenosis who have been determined by a cardiac surgeon to be inoperable for open aortic valve replacement and in whom existing co-morbidities would not preclude the expected benefit from correction of the aortic stenosis.
The FDA’s approval of the Sapien is based on a study in 365 patients who were not eligible for open-heart surgery. Half of the patients received the Sapien valve. The other study patients received another treatment that did not require open-heart surgery. One alternative procedure involved enlarging the aortic valve opening by stretching it with a balloon (balloon valvuloplasty).
Patients receiving the Sapien valve experienced two-and-a-half times more strokes and eight times as many vascular and bleeding complications than patients who did not receive the implant; however, they were more likely to survive one year after surgery. After a year, 69 percent of the Sapien patients were alive, compared with 50 percent of those who received an alternative treatment.
Edwards Lifescience, the manufacturer of the Sapien, will continue to evaluate the outcomes with the Sapien through a national Transcatheter Valve Therapy (TVT) registry. The Society of Thoracic Surgeons (STS) and the American College of Cardiology (ACC) have been working with the FDA and the Centers for Medicare and Medicaid Services (CMS) to facilitate the creation of the national TVT registry that will serve as a platform for continued evaluation of post-market experience with this and future transcatheter devices and procedures for the treatment of aortic stenosis.
The most common serious and potentially life-threatening side effects in patients receiving the Sapien valve and the procedure to implant the valve include death, stroke, perforation of the blood vessels, ventricle or valvular structures, damage to the conduction system in the heart, significant bleeding and leaks around the new valve.
The Sapien is approved for patients who are not eligible for open-heart surgery for replacement of their aortic valve and have a calcified aortic annulus (calcium buildup in the fibrous ring of the aortic heart valve). The product label advises that a heart surgeon should be involved in determining if the Sapien is an appropriate treatment for the patient.
It is not approved for patients who can be treated by open-heart surgery. Patients who have congenital heart valve anomalies, have masses or an infection in their hearts, or cannot tolerate anticoagulation/antiplatelet therapy should not receive the Sapien.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









