October 14, 2011 — GE Healthcare received U.S. Food and Drug Administration (FDA) clearance of the Discovery MR750w wide bore 3.0T system with GEM (Geometry Embracing Method) suite of coils.
This new wide bore 3.0 Tesla magnetic resonance (MR) system addresses patient demand for a better and more comfortable scanning experience. In particular, the new patient-friendly design accommodates patients who are usually difficult to scan; this includes larger, claustrophobic, elderly or very young patients, or those who are in pain and require a larger imaging system.
Additionally, the system combines next-generation clinical applications and a full 50 x 50 x 50 cm field of view. It helps reduce exam time and scan large anatomies with fewer scans, compared to previous generation systems. It also enables higher patient throughput and satisfaction.
The system provides the power of a 3.0T magnet with the open architecture of a 70 cm wide bore. Through fully automated and independent radiofrequency (RF) pulse amplitude and phase control, MultiDrive RF Transmit produces consistently clear 3.0T images.
The system’s gradient and RF performance is optimized to shorten TR’s and TE’s to produce sharp and clear images.
GE’s Optical RF (OpTix) offers high channel count, analog to digital-optical signal conversion inside the scan room to minimize noise and signal degradation but away from the patient to enhance comfort.
Advances in RF coil design are possible through the use of thinner, lightweight, flexible material applicable with a variety of body types, allowing easier patient positioning. Crafted to embrace the patient, these flexible coils make for a more relaxed scan experience. This also makes it easier for technologists to correctly position their patients without strain or difficulty.
The GEM Suite includes a high density GEM Posterior Array embedded within the detachable Express patient table, the Head and Neck Unit with comfort tilt, the Anterior Array, and the Peripheral Vascular/Lower Extremity Array. Covering 98 percent of all exam types, the coils can be used individually or combined to provide head to toe patient coverage.
The suite enables automatic configuration that best fits the selected region of interest. It includes a total 205 cm scanning range, feet first or head first scanning for all anatomies, and design features to embrace patients of a wide variety of shapes and sizes.
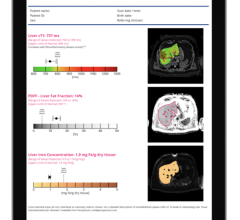
IDEAL IQ provides a quantitative fat content assessment in the entire liver, while MR Touch is an MR elastography-based imaging technique evaluating tissue stiffness.
For more information: www.gehealthcare.com


 January 21, 2026
January 21, 2026