April 1, 2011 – A new minimally invasive embolization catheter was unveiled at the Society of Interventional Radiology Annual Scientific Meeting. The Cantata Superselective Microcatheter, from Cook Medical, offers a combination of support and torqueability to help interventional radiologists navigate tortuous anatomies.
Additionally, it minimizes trauma with distal softness and microwire tracking.
“Interventional radiologists are at the forefront of advancing minimally invasive procedures and play a critical role in improving patient outcomes,” said Dan Sirota, vice president and global leader of Cook Medical’s Interventional Radiology division. “As a leader in embolic therapies, Cook Medical is proud to provide interventional radiologists with tools like Cantata to advance their field of medicine.”
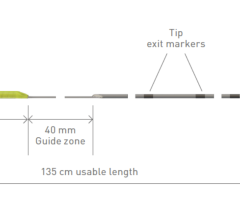
Accepting 0.018 inch embolization coils, the Cantata allows coil delivery and can be used in combination with a wide range of emobolization products, including the cmopany’s MicroNester and Tornado microcoils. Designed to be Lipiodol and dimethyl sulfoxide (DMSO) compatible, the microcatheter can be used in a range of procedures. The kink-resistant braided construction along the entire length of the shaft provides improved torque response and trackability for vessel selection while maintaining catheterization.
With a hydrophilic coating designed to reduce surface friction, the 2.8 and the 2.5 French catheter lines can achieve a flow rate as high as 3.5 mL/sec and 1.6 mL/sec, respectively. The five durometer zones, more than any other microcatheter, deliver a distinct yet seamless transition from the shaft to the soft, distal tip, reducing the risk of trauma.
The device received U.S. Food and Drug Administration clearance in December of 2010.
For more information: www.cookmedical.com


 January 29, 2026
January 29, 2026