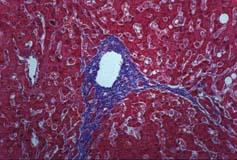
Galectin-3 is a mediator of cardiac fibrosis (pictured) and adverse remodeling in heart failure patients.
March 25, 2011 – A new galectin-3 test, recently cleared by the U.S. Food and Drug Administration (FDA) to help assess the prognosis of patients with chronic heart failure, is now offered by Health Diagnostic Laboratory (HDL). BG Medicine Inc. announced the agreement under which HDL will offer galectin-3 testing services based on the BG Medicine Galectin-3 Test.
Elevated galectin-3 levels are associated with an inherently progressive form of heart failure that is associated with an increased risk of hospitalization or death. Galectin-3 has been implicated in a variety of biological processes important in heart failure development and progression, including fibrosis. Progressive cardiac fibrosis interferes with the cardiac pump function and increases the risk of death.
"HDL Inc. is providing a new model of laboratory testing services to over 5,000 physicians with an emphasis on markers related to cardiovascular disease," said Pieter Muntendam, M.D., president and CEO of BG Medicine. "The cardiovascular focus of HDL, Inc. makes this an excellent partnership to bring our message of the importance of galectin-3 in cardiac disease to the growing number physicians served by HDL Inc."
"Galectin-3 further enhances our extensive cardiovascular disease offering," said Tonya Mallory, CEO and co-founder of HDL Inc. "Novel markers like galectin-3 are expected to enable a new medical model where physicians can triage patients not only based on their clinical status on the day of the visit, but also based on the type of underlying disease process and its prognostic implications."
For more information: www.myhdl.com, www.galectin-3.com

 October 09, 2019
October 09, 2019