February 21, 2011 – Scottsdale Healthcare Shea Medical Center in Arizona is the first hospital in the southwestern United States to implant a magnetic resonance imaging (MRI)-safe pacemaker. This represents a major technological breakthrough for patients who need MRI diagnostic scans, which can damage older style pacemakers or cause serious health complications.
A pacemaker is a small device placed in the chest to help control abnormal heart rhythms, using electrical pulses to prompt the heart to beat at a normal rate. Nearly 200,000 pacemaker patients in the United States annually are not eligible for MRI scans, which are critical for making a wide range of health diagnoses.
Up to 75 percent of patients with electronic cardiac devices will likely need an MRI over their device's lifetime, according to Medtronic, a pacemaker manufacturer.
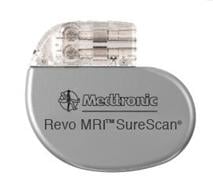
Thomas Mattioni, M.D., successfully placed the device in an 81-year-old patient at Scottsdale Healthcare Shea Medical Center. Mattioni is the medical director of electrophysiology at Scottsdale Healthcare. The Medtronic Revo MRI SureScan heart pacemaker received approval from the U.S. Food and Drug Administration on Feb. 8.
"MRI-safe pacemakers allow improved detection and treatment of serious medical conditions such as stroke, cancer and a wide variety of important neurologic and orthopedic conditions," Mattioni said. Previously, these patients either did not get an MRI, or were exposed to higher radiation CT scans.
Magnetic resonance imaging (MRI) exposure can interfere with pacemaker operation, damage the device or change the pacing capture threshold, the minimum amount of electrical current required to evoke a cardiac contraction.
Mattioni said that prior to the introduction of the MRI-safe pacemaker, patients could face serious complications if exposed to the powerful magnetic fields generated by MRI machines. They can be as much as 30,000 times more powerful than the Earth's magnetic field.
"Patients at Scottsdale Healthcare now have access to a state-of-the-art pacemaker that is designed to work safely and effectively in an MRI environment," Mattioni said. "This new pacemaker technology can provide a meaningful difference in patients' lives."
The Revo MRI pacemaker is considered MR-Conditional, a term used to indicate that a device may be used in the MRI environment under certain conditions, such as a particular type of MRI scanner and scanner settings.
MRI scanners may cause other current pacemakers to misinterpret MRI-generated electrical noise and withhold pacing therapy or deliver unnecessary pacing therapy. This new pacemaker includes a feature that sets the device into an appropriate mode for the MRI environment. This new pacing system also includes specially designed leads that protect the patient from heart damage that can occur if MRI is performed in currently available pacing systems.
For more information: www.shc.org


 January 21, 2026
January 21, 2026