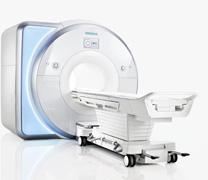
October 8, 2010 – Two new magnetic resonance imaging (MRI) scanners have received 510(k) FDA clearance and are now commercially available in the United States. The Siemens Magnetom Aera 1.5 Tesla and Magnetom Skyra 3T scanners are the first Tim 4G and DOT systems to receive U.S. clearance.
Tim 4G, the fourth generation of Siemens-exclusive RF technology, starts with 48 RF channels at both 1.5 and 3T – presently the highest standard RF channel configuration offered at either field strength. Tim’s new all digital-in/digital-out design, DirectRF, integrates all RF transmit and receive components at the magnet, eliminating analog cables.
Additionally, a new coil architecture, Dual-Density Signal Transfer, sends signals from two independent coil elements on a single wire using two different frequencies. This offers improved signal-to-noise and enables higher density of RF elements/channels within a given coil housing.
The Aera and Skyra systems also feature the DOT (day optimizing throughput) engine that provides a customizable framework for patient personalization, user guidance and exam automation.
The Magnetom Aera and Skyra systems have been available outside the United States since November 2009.
For more information: www.siemens.com/healthcare


 January 21, 2026
January 21, 2026